Session Information
Date: Sunday, November 12, 2023
Title: (0066–0095) T Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
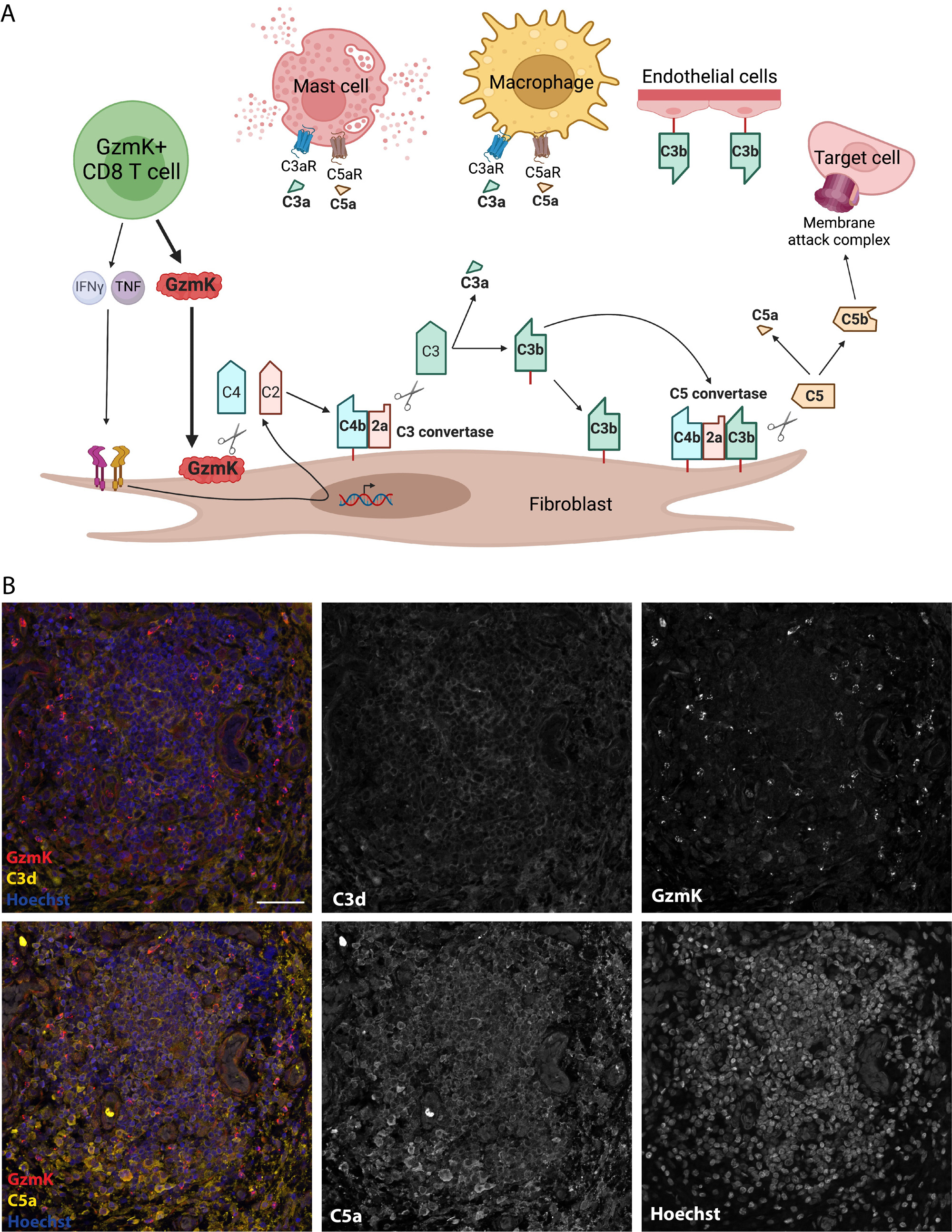
Background/Purpose: T cells are major drivers of rheumatoid arthritis (RA) pathogenesis. While most research has focused on CD4+ T cells, we have found that CD8+ T cells – specifically granzyme K (GzmK)-expressing CD8 T cells – are strikingly abundant in inflamed RA synovium as well as other tissues affected by autoimmune disease. Unlike granzyme B (GzmB), GzmK does not cleaves caspases to induce target cell apoptosis, and the function of GzmK in inflamed synovium and other tissues is unclear. Here, we describe that GzmK is continuously released from CD8+ T cells and activates a new complement pathway, opsonizing target cells and releasing anaphylatoxins C3a and C5a that recruit and activate innate immune cells. In inflamed RA synovium, we find complement fragments C3d and C5a in proximity to GzmK+ CD8 T cells, supporting a role for this complement activation mechanism in vivo.
Methods: We incubated recombinant enzymatically active GzmK with serum-purified complement fragments to investigate the molecular effects of GzmK on complement proteins. We measured cleavage of C2, C3, C4, and C5 using Western blots. We used flow cytometry to assess C3b and terminal complement complex (TCC) deposition on cell membranes. We performed multicolor immunofluorescence microscopy of human rheumatoid arthritis synovial tissue to assess complement activation and deposition relative to GzmK+ CD8+ T cells.+
Results: GzmK is expressed by a wide range of T cells and innate-like lymphocytes in human synovial tissue and fluid in RA and in other autoimmune and inflamed tissues. Resting GzmK+ CD8+ T cells continuously release GzmK, which binds to negatively charged residues on cell surfaces. Synovial fibroblasts express complement components C2, C3, and C4, and this production is amplified by T cell-associated cytokines IFNγ and TNF. GzmK cleaves C4 to C4b and C2 to C2a to form an active C3 convertase (C4b + C2a) that cleaves C3 to C3a and C3b, which covalently deposits on cell membranes due to the surface localization of GzmK. These complement fragments then form the C5 convertase C4b2b3b, ultimately leading to release of biologically active anaphylatoxin C5a and surface deposition of the terminal complement complex (C5b-C9) (Figure 1A). Using multicolor immunofluorescence microscopy, we find complement products C3/C3d and C5a in proximity to GzmK+ CD8+ T cells in inflamed RA synovium (Figure 1B).
Conclusion: GzmK activates a novel complement pathway that is independent of the classical, alternative, and lectin pathways of complement activation. GzmK can cleave locally generated complement components to generate C3a, C3b, C5a, and terminal complement complex, which promote inflammatory cell recruitment and antigen uptake. In inflamed RA synovium, C3/C3d and C5a deposition occurs near GzmK+ cells, supporting the in vivo relevance of this new mechanism of complement activation. Together, these findings reveal a new pro-inflammatory in RA that is likely active in other autoimmune diseases as well.
To cite this abstract in AMA style:
Jonsson A, Donado C, Theisen E, Jones D, Nathan A, Zhang F, Medicines Partnership (AMP): RA/SLE A, Raychaudhuri S, Brenner M. Granzyme K Elicits a New Pathway for Complement Activation in RA Synovium [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/granzyme-k-elicits-a-new-pathway-for-complement-activation-in-ra-synovium-2/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/granzyme-k-elicits-a-new-pathway-for-complement-activation-in-ra-synovium-2/