Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Synovial fibroblasts are a promising therapeutic target in rheumatoid arthritis (RA) where they can adopt inflammatory or destructive phenotypes and directly promote bone and cartilage degradation. In our prior analyses, we found that non-canonical Wnt activation induces robust inflammatory gene expression in these cells. Moreover, two Wnt modulators, RSPO3 and DKK3, are reciprocally expressed and form a transcriptional gradient associated with increased and decreased Wnt activation, respectively. Strikingly, RSPO3 and Wnt activation signatures are enhanced in synovial fibroblasts in RA, suggesting the potential relevance of the Wnt pathway in vivo. Here, we explore the clinical impact of Wnt signaling in mouse models of inflammatory arthritis where we demonstrate that Wnt activation worsens the severity and prolongs the course of arthritis.
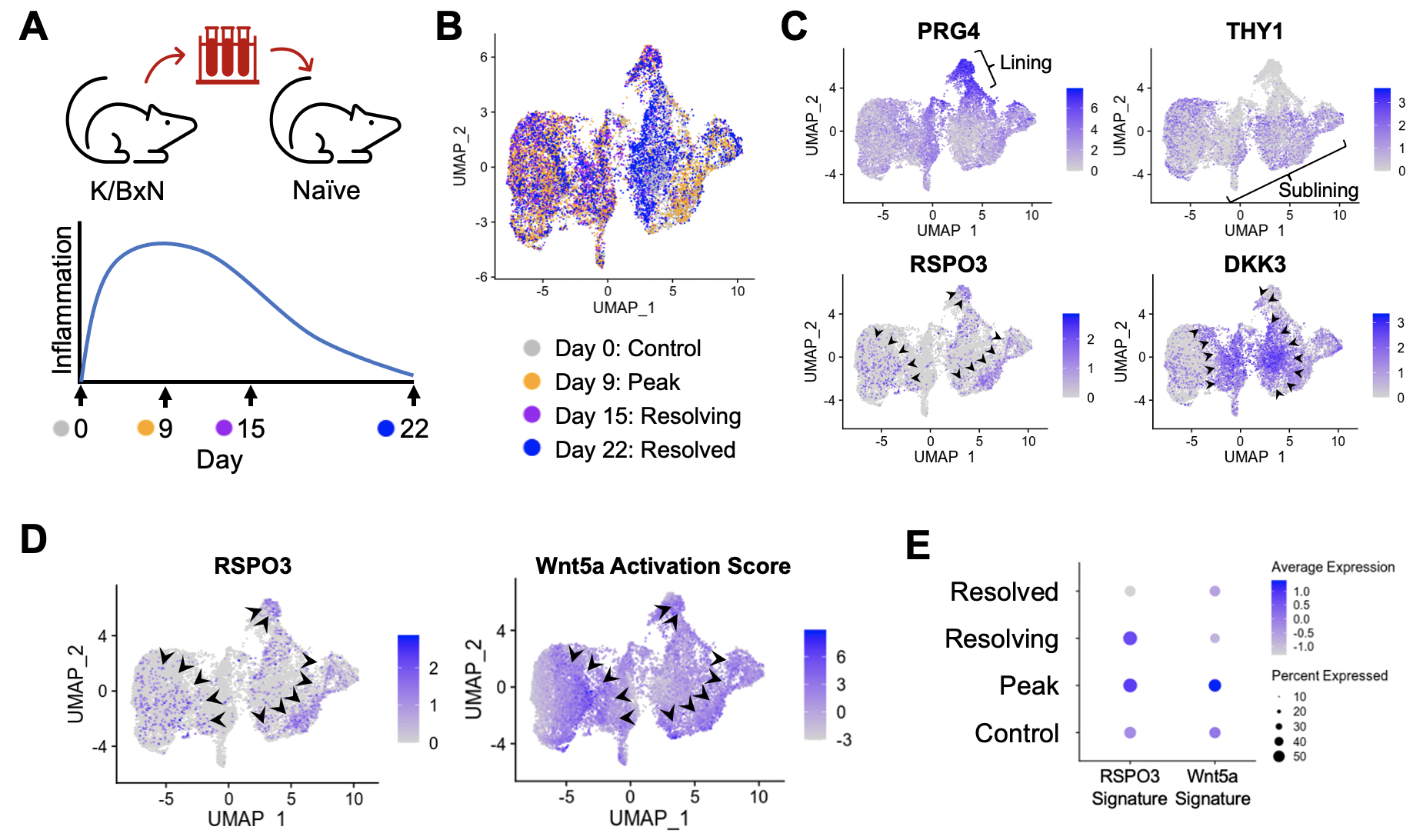
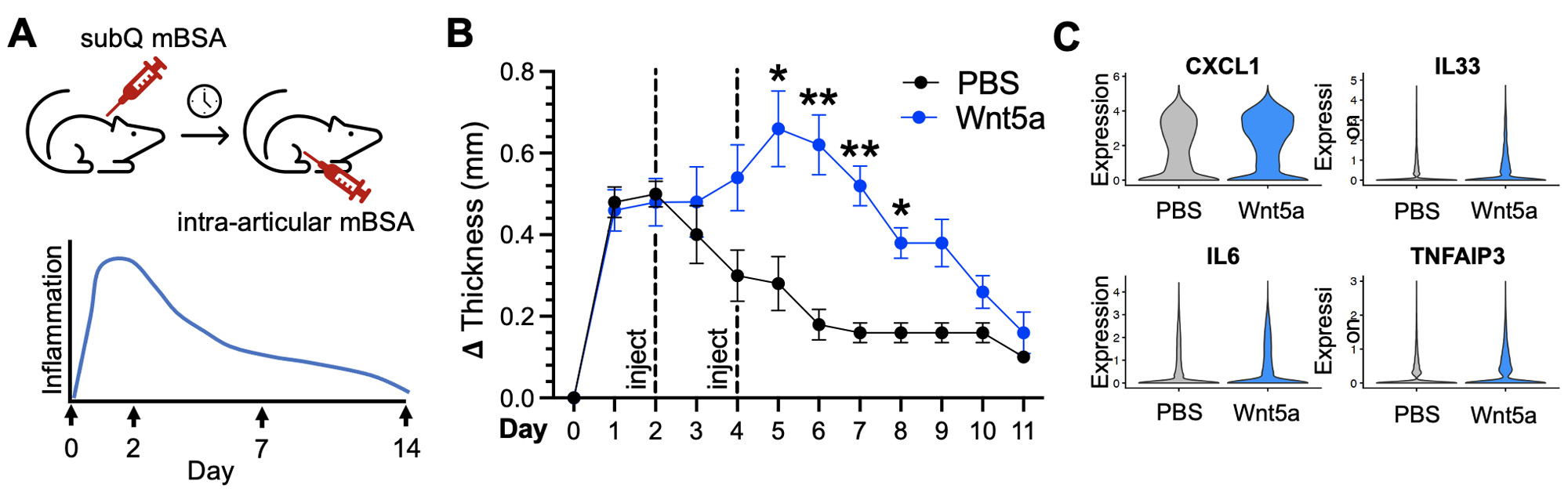
Methods: Single-cell RNA sequencing (scRNA-seq) libraries were generated from enzymatically digested, sort-purified CD45- synovial tissue dissected from K/BxN serum recipient mice at the indicated time points. To generate Wnt activation signatures, human RA synovial fibroblasts were stimulated in vitro with a combination of Wnt ligands, doses, and time points, and we performed RNA-seq and differential gene expression analysis. RSPO3 signatures were developed from scRNA-seq data of synovial fibroblasts from the Roche Fibroblast Network Consortium. In the antigen-induced arthritis (AIA) model, C57Bl/6 mice underwent subcutaneous (day -21) and knee intra-articular (day 0) injections of methylated BSA (mBSA) followed by knee intra-articular injections of PBS or Wnt5a (days 2 and 4). Knee diameters were measured using calipers, and synovial samples were collected at day 7 for scRNA-seq.
Results: Synovial tissues were processed at baseline, peak inflammation, resolving and resolved time points in K/BxN serum transfer recipient mice (Figure 1A). scRNA-seq analyses reveal reciprocal expression of RSPO3 and DKK3, similar to the pattern in human RA synovium (Figure 1B and 1C). Likewise, Wnt activation scores are enriched in cells that show high expression of the Wnt agonist RSPO3 (Figure 1D). Strikingly, RSPO3 and Wnt activation signatures are increased in synovial fibroblasts isolated at the peak of arthritis compared to those from control and resolution time points (Figure 1E). To determine how the activation of Wnt signaling would impact clinical arthritis, we used the AIA model where non-canonical Wnt5a ligand could be directly injected into the knee (Figure 2A). Strikingly, we found that Wnt activation induces a profound increase in the severity and duration of arthritis (Figure 2B) associated with increased expression of cytokines, chemokines, and other inflammatory mediators in synovial fibroblasts (Figure 2C).
Conclusion: Here, we demonstrate that stromal activation of Wnt signaling identified in human RA is recapitulated in mouse models of inflammatory arthritis and that non-canonical Wnt activation is associated with worsening severity of clinical arthritis. This work provides evidence supporting a new molecular target for treatments directed at synovial fibroblasts in RA.
To cite this abstract in AMA style:
Mueller A, Zou A, Marsh L, Kemble S, Nayar S, Taylor E, Major T, Gardner D, Watts G, Murphy C, Fibroblast Network Consortium R, Croft A, Filer A, Buckley C, Wei K, Korsunsky i, Raychaudhuri S, Brenner M. Wnt Signaling Drives Pathogenic Stromal Inflammation in Inflammatory Arthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/wnt-signaling-drives-pathogenic-stromal-inflammation-in-inflammatory-arthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/wnt-signaling-drives-pathogenic-stromal-inflammation-in-inflammatory-arthritis/