Session Information
Date: Tuesday, October 28, 2025
Title: (2470–2503) Systemic Sclerosis & Related Disorders – Clinical Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
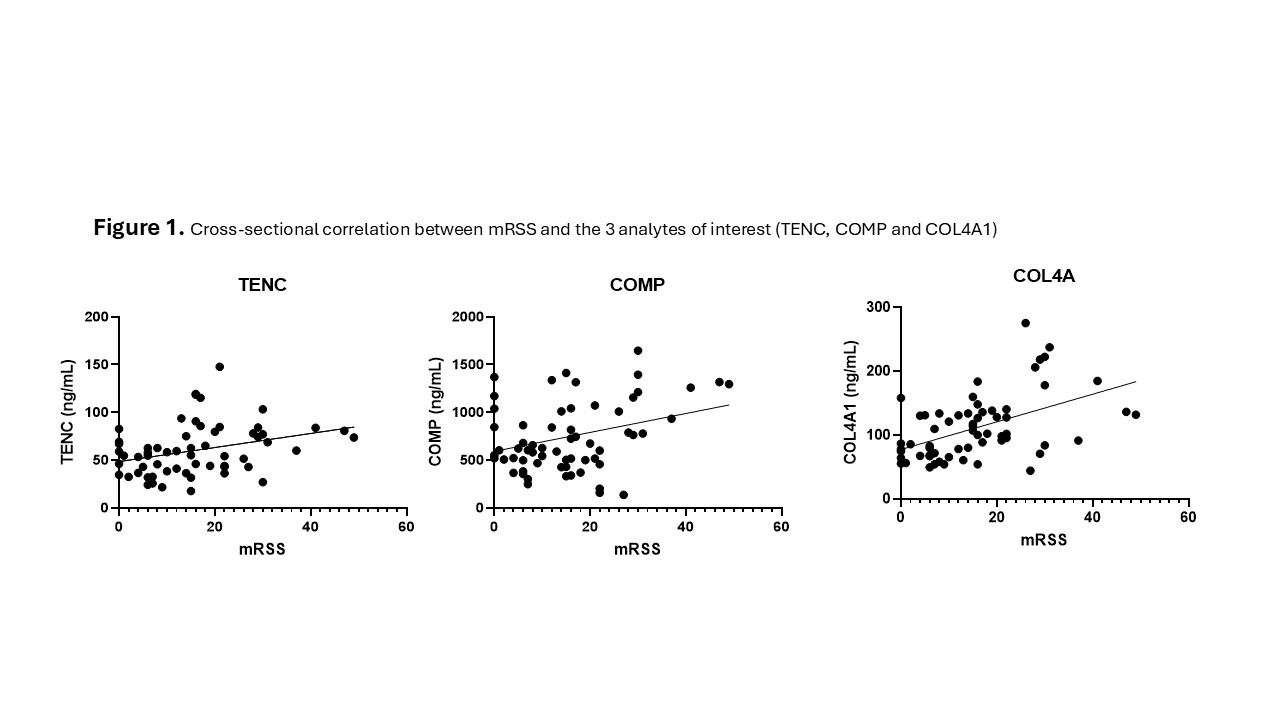
Background/Purpose: Skin fibrosis is a cardinal manifestation of diffuse cutaneous systemic sclerosis (dcSSc) and is routinely measured via the modified Rodnan skin score (mRSS); however, mRSS is limited by its operator-dependence and may not reflect the complex disease biology at the cutaneous compartment. A recent high dimensional multi-omic analysis identified 3 serum proteins independently associated with mRSS: tenascin C (TNC), cartilage oligomeric matrix protein (COMP), and collagen type IV alpha 1 (COL4A1). Integrating their serum concentrations, a composite biomarker score to reflect skin involvement was derived and tested in 2 cohorts from a single centre. Whilst there was correlation of the composite marker and its components with mRSS and some association with underlying treatment, the results of these studies were inconclusive, potentially due to the narrow range of mRSS and modest impact of standard immunosuppressive treatment in the SSc cohorts. The aim of the present study was to validate this composite biomarker score in a cohort with a greater dynamic range of mRSS and high percentage of patients receiving intensive immunosuppressive treatment.
Methods: We selected patients from 2 centres in the Netherlands who participated in the Autologous Stem Cell Transplantation International Scleroderma (ASTIS) trial. Serum concentrations of TNC, COMP and COL4A1 were measured via commercially available enzyme linked immunosorbent assay (ELISA) kits on samples at baseline, 12 months and 24 months. Serum levels of target analytes were correlated with mRSS data cross-sectionally and longitudinally. Spearman’s test, Bland Altman test, univariable and multivariable linear regression were used to test for correlation. A p value < 0.05 was considered statistically significant.
Results: We included 21 patients: 11 treated with cyclophosphamide (CYC), and 10 subjected to autologous haematopoietic stem cell transplant (HSCT). Median mRSS was 21 at baseline, 12 at 12 months, and 6 at 24 months. The concentrations of all 3 analytes showed a significant correlation with mRSS when evaluated cross-sectionally at all the timepoints (Figure 1), with COL4A1 maintaining statistical significance at multivariable analysis (r=0.0952, p=0.001). From these data, we derived a refined composite serum biomarker score to predict actual mRSS (Figure 2). Next, we assessed the performance of the analytes as dynamic biomarkers of mRSS, however only changes in COMP were associated with mRSS variation (r= 0.013; p= 0.012). Finally, although both treatment arms had the same degree of mRSS variation from baseline to 24 months (i.e. 15 points), the change in COL4A1 concentration was significantly larger in the HSCT group than in the CYC group (Figure 3).
Conclusion: Our refined composite biomarker score predicts mRSS score cross-sectionally, however the performance of these analytes as dynamic markers of cutaneous involvement is poor. The differences for serum COL4A1 across treatment groups, with greater reduction in the HSCT group, was associated with a long-term survival advantage in the ASTIS trial which warrants further evaluation of the role of this protein as a predictive marker in dcSSc
To cite this abstract in AMA style:
rodolfi s, clark k, Ahmed Abdi b, kanitkar m, Ong V, Voskuijl A, de Vries-Bouwstra J, van Laar J, Denton C, Spierings j. Validation of a Composite Biomarker Score To Predict Modified Rodnan Skin Score: Insight From Autologous Stem Cell Transplantation International Scleroderma trial [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/validation-of-a-composite-biomarker-score-to-predict-modified-rodnan-skin-score-insight-from-autologous-stem-cell-transplantation-international-scleroderma-trial/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validation-of-a-composite-biomarker-score-to-predict-modified-rodnan-skin-score-insight-from-autologous-stem-cell-transplantation-international-scleroderma-trial/

.jpg)
.jpg)