Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients with systemic sclerosis (SSc) frequently experience gastrointestinal (GI) symptoms, ranging from mild to debilitating in severity. Better prediction of those most at risk of developing severe GI disease would be helpful for clinical trials and practice.
Previously, we identified demographic factors and serological markers associated with certain gastrointestinal symptoms, in a cohort (n=214) of consecutive SSc patients. Here, we have validated and extended our work in a second independent cohort.
Methods: SSc patients attending our centre between December 2019 and March 2020 completed the UCLA SCTC GIT 2.0 questionnaire, encompassing various domains of SSc-associated GI disease (reflux, distension/bloating, diarrhoea, soilage, constipation, social functioning and emotional wellbeing). Higher domain scores correspond with greater GI disease severity (domain scores range from 0 to 3). Analysis of associations was undertaken using appropriate non-parametric tests. Subgroups on basis of antibodies included ACA, ATA, ARA, U3RNP, nRNP, PmScl and other antibodies. Double autoantibodies were present in 10 subjects and those were classified into the group of the more SSc-specific antibody.
Results: 314 patients completed UCLA GIT 2.0. Mean (SD) age was 55.2 (14.5) and 85.7% were female. The mean number of years since SSc onset was 12.9 (10.0). Limited cutaneous SSc was present in 66.6%, and disease overlap syndromes were present in 25.4%. The most common autoantibody was anti-centromere (ACA, 28.0%), followed by anti-topoisomerase (ATA, 24.2%), anti-RNA polymerase III (ARA, 9.9%), Ro (8.9%), anti-PMScl (6.1%) and anti-nRNP (5.7%). The mean (SD) total GIT score was 0.70 (0.58) and 56.4% patients reported moderate-severe symptoms (Table 1). There was a positive correlation between total GIT score and patient visual analogue scale (VAS) self-reported GI disease burden (Spearman’s Rho = 0.68, p < 0.0001). There was no association between GI symptoms and disease subset or duration. Gender was only associated with emotional wellbeing, which was more severely affected in females compared to males (score 0.68 v 0.42, p=0.0339). There were significant differences between the antibody subgroups in terms of GI symptoms (Table 2). Of the SSc-specific antibodies, ARA and ACA appeared to convey increased risk overall and throughout the domains, demonstrating the highest scores. Mean/median total GIT scores and scores for reflux, distension/bloating, constipation, social functioning and emotional wellbeing were highest among ARA+, while scores for diarrhoea and faecal soilage were highest among ACA+ subjects. On the other hand, anti-PmScl+ patients had the lowest scores in most domains, including total GIT 2.0 score.
Conclusion: We have validated our previous results in a second SSc cohort. Higher GIT scores were associated with significantly worse self-reported impact on quality of life. In addition, in this cohort, ARA and ACA positivity appeared to be associated with more severe GI disease. Future studies should explore whether ANA reactivity may identify cases at risk of severe GI manifestations.
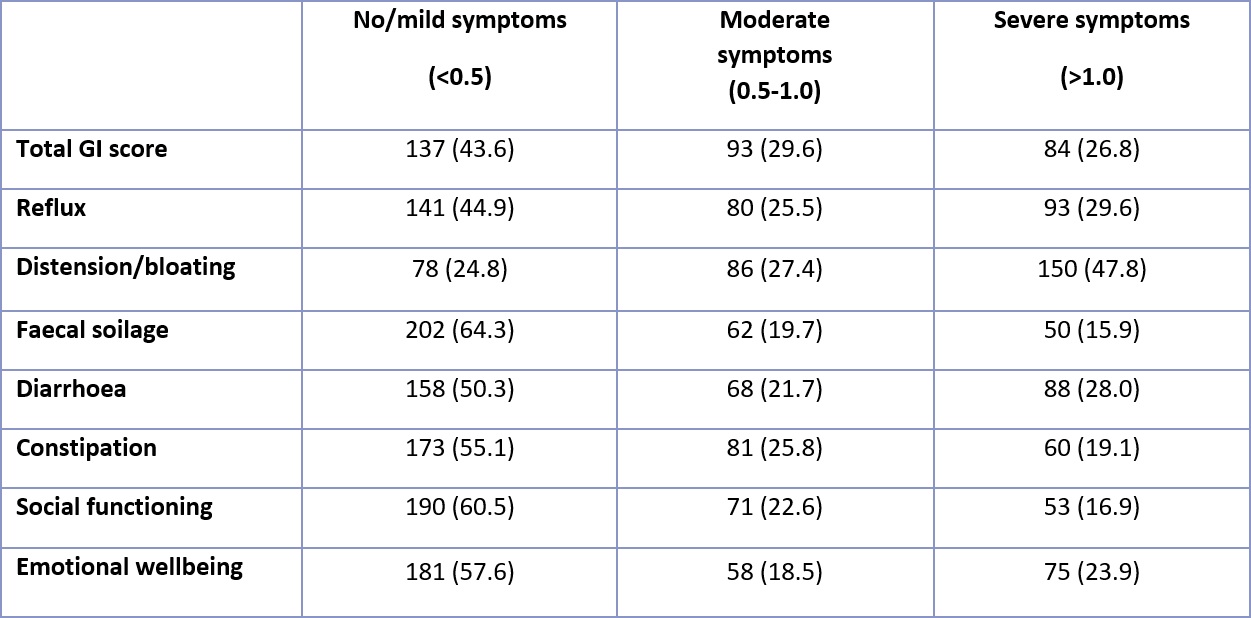 Table 1. Distribution of severity of GI symptoms in SSc cohort (n=314), based on total GI score and individual domain scores for patients fully completing the UCLA SCTC GIT 2.0 questionnaire. Total GI score excludes constipation score. Total score and individual domain score frequencies are represented as n (%).
Table 1. Distribution of severity of GI symptoms in SSc cohort (n=314), based on total GI score and individual domain scores for patients fully completing the UCLA SCTC GIT 2.0 questionnaire. Total GI score excludes constipation score. Total score and individual domain score frequencies are represented as n (%).
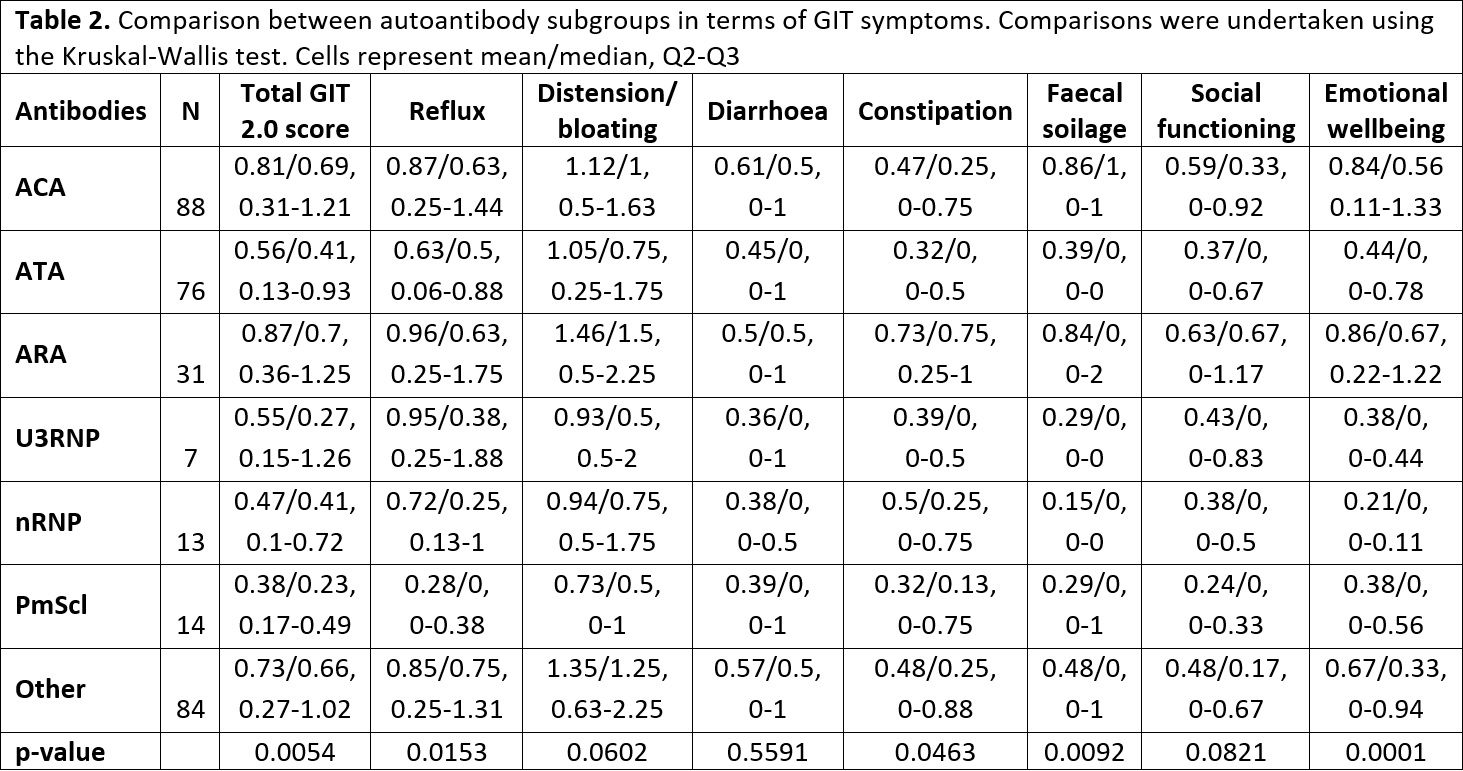 Table 2. Comparison between autoantibody subgroups in terms of GIT symptoms. Comparisons were undertaken using the Kruskal-Wallis test. Cells represent mean/median, Q2-Q3.
Table 2. Comparison between autoantibody subgroups in terms of GIT symptoms. Comparisons were undertaken using the Kruskal-Wallis test. Cells represent mean/median, Q2-Q3.
To cite this abstract in AMA style:
Ahmed F, Nihtyanova S, Chatzinikolaou S, Ong V, Murray C, Denton C. Validating Autoantibody Associations and Clinical Impact of Severe Gastrointestinal Involvement in Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/validating-autoantibody-associations-and-clinical-impact-of-severe-gastrointestinal-involvement-in-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validating-autoantibody-associations-and-clinical-impact-of-severe-gastrointestinal-involvement-in-systemic-sclerosis/
