Session Information
Session Type: Abstract Session
Session Time: 5:00PM-5:50PM
Background/Purpose: Oligoarticular juvenile idiopathic arthritis (oligo JIA) is defined by limited joint involvement at disease onset. Some children achieve long-term remission while others continue to have chronic arthritis in a few joints or extend to a polyarticular course. The mechanisms driving persistent or extended disease in a subgroup of patients are unknown. To better understand how CD4+ T cell responses evolve in oligo JIA, we characterized regulatory (Treg) and effector (Teff) T cells found in the joints of patients at disease onset and during recurrent arthritic flares.
Methods: Synovial fluid (SF) and paired peripheral blood (PB) samples were collected from oligo JIA patients, defined by ILAR criteria. PB was also obtained from healthy controls (HC). Tregs (CD3+CD4+CD25+CD127lo) and Teffs (CD3+CD4+CD25–) were sorted from SF and PB, and processed for bulk RNA sequencing, single-cell RNA sequencing coupled with T cell receptor (TCR) repertoire analysis (10x genomics), DNA methylation studies (EpigenDx), or in vitro Treg suppression assays. PB and SF mononuclear cells were evaluated with flow cytometry.
Results: 34 oligo JIA patients and 15 controls were studied. In SF, flow cytometry demonstrated significantly increased frequencies of memory CD4+ T cells (CD3+CD4+CD45RO+) expressing Th1 related cytokines (IFNγ) and chemokine receptors (CXCR3). Th17 cells were not enriched in the joint. SF Tregs (CD4+CD25+CD127loFoxp3+) were markedly skewed to a Th1 phenotype: 76.3% ± 6.0% (SEM) expressed CXCR3, and 3.2% ± 0.4% secreted IFNγ after stimulation in vitro (Fig1). Bulk RNA sequencing confirmed a Th1 signature in SF Tregs and SF Teffs at disease onset and relapse, with elevated levels of TBX21 (T-bet, master regulator of the Th1 fate), CXCR3 and IFNG transcripts in oligo JIA SF compared to oligo JIA PB and HC PB. Gene set enrichment analysis uncovered IFNγ signaling as one of the most enriched pathways in SF Tregs and SF Teffs (Fig2). The Treg transcriptional signature was preserved in SF Tregs. In addition, FOXP3 locus demethylation and Treg suppressive capacity was maintained in Th1-like Tregs (CXCR3+ Tregs), confirming the regulatory identity of this population. Single-cell RNA sequencing uncovered heterogeneity in oligo JIA SF Tregs, with 5 distinct subsets: (1) ‘classical’ Tregs, (2) IL2RAlow Tregs, (3) activated, HLA-DRhi Tregs, (4) Th1-like Tregs expressing FOXP3 and IL2RA jointly with TBX21 and IL12RB2, and (5) Tregs expressing other IFNγ induced genes. Except for IL2RAlo Tregs, all other Treg clusters were enriched in expanded TCR clones, with 22.0 to 37.0% of cells per cluster having at least 3 clonal copies across the single-cell dataset.
Conclusion: Our results identify a strong Th1 polarization in the joints of oligo JIA patients that encompasses both regulatory and effector CD4+ T cells and persists longitudinally. While the majority of SF Tregs display Th1 features, this population is heterogeneous with at least 5 subpopulations. Th1-like Tregs retain their regulatory gene expression signature, methylation patterns and functional capacity. These results suggest that augmenting the function of Th1-like Tregs may be key in controlling Th1 mediated inflammation in the joint and restoring tolerance in oligo JIA.
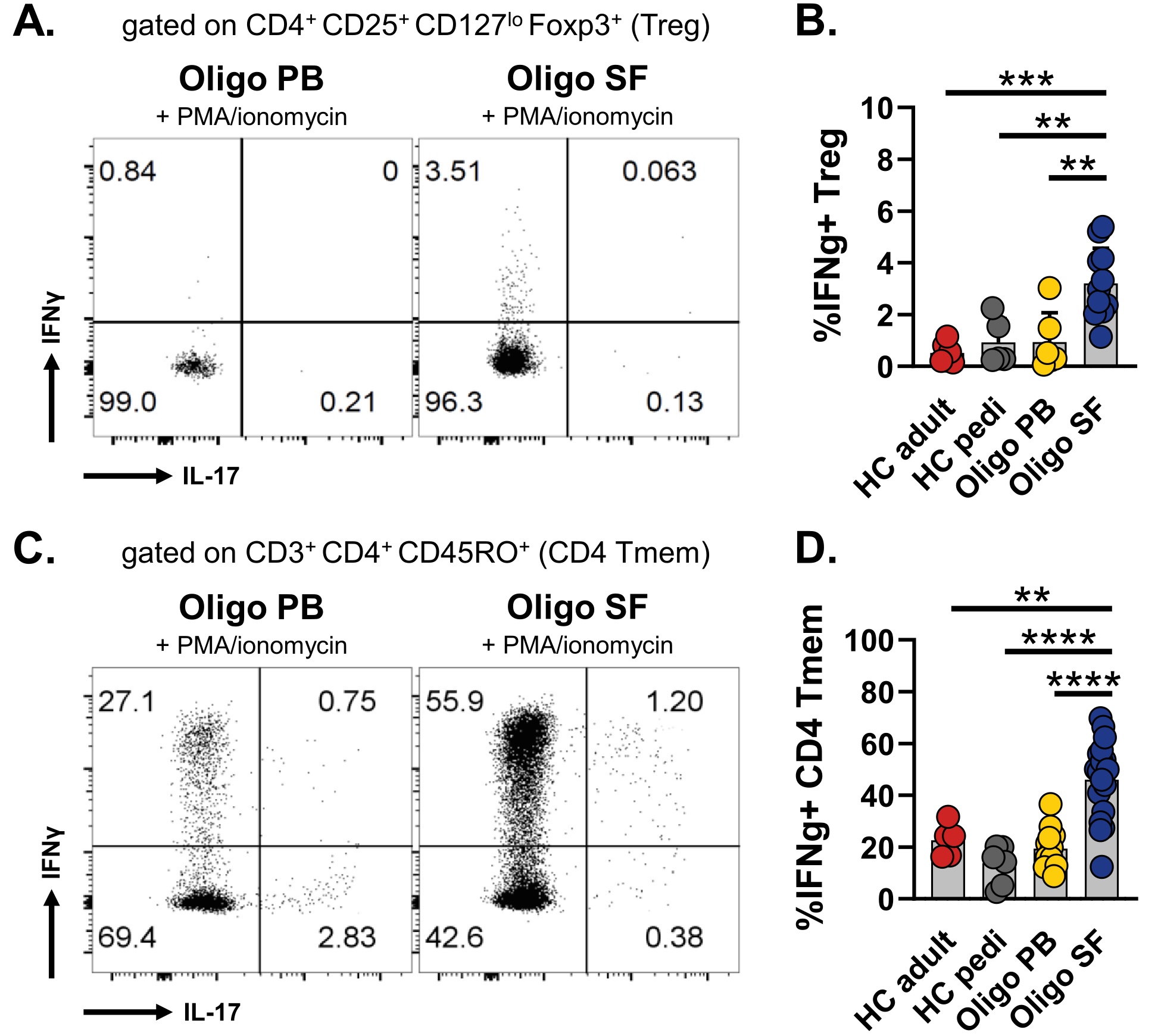 CD4+ T cells from oligo JIA SF express IFNγ upon stimulation in vitro. A) and C) Representative flow cytometry dot plots for Tregs (A) and memory CD4+ T cells (C). B) and D) Mean frequencies +SD of IFNγ+ Tregs (B) and memory CD4+ T cells (D), as measured by flow cytometry (ANOVA with correction for multiple comparisons, **p < 0.01, ***p < 0.001, ****p < 0.0001). PB, peripheral blood; SF, synovial fluid; HC, healthy control; pedi, pediatric.
CD4+ T cells from oligo JIA SF express IFNγ upon stimulation in vitro. A) and C) Representative flow cytometry dot plots for Tregs (A) and memory CD4+ T cells (C). B) and D) Mean frequencies +SD of IFNγ+ Tregs (B) and memory CD4+ T cells (D), as measured by flow cytometry (ANOVA with correction for multiple comparisons, **p < 0.01, ***p < 0.001, ****p < 0.0001). PB, peripheral blood; SF, synovial fluid; HC, healthy control; pedi, pediatric.
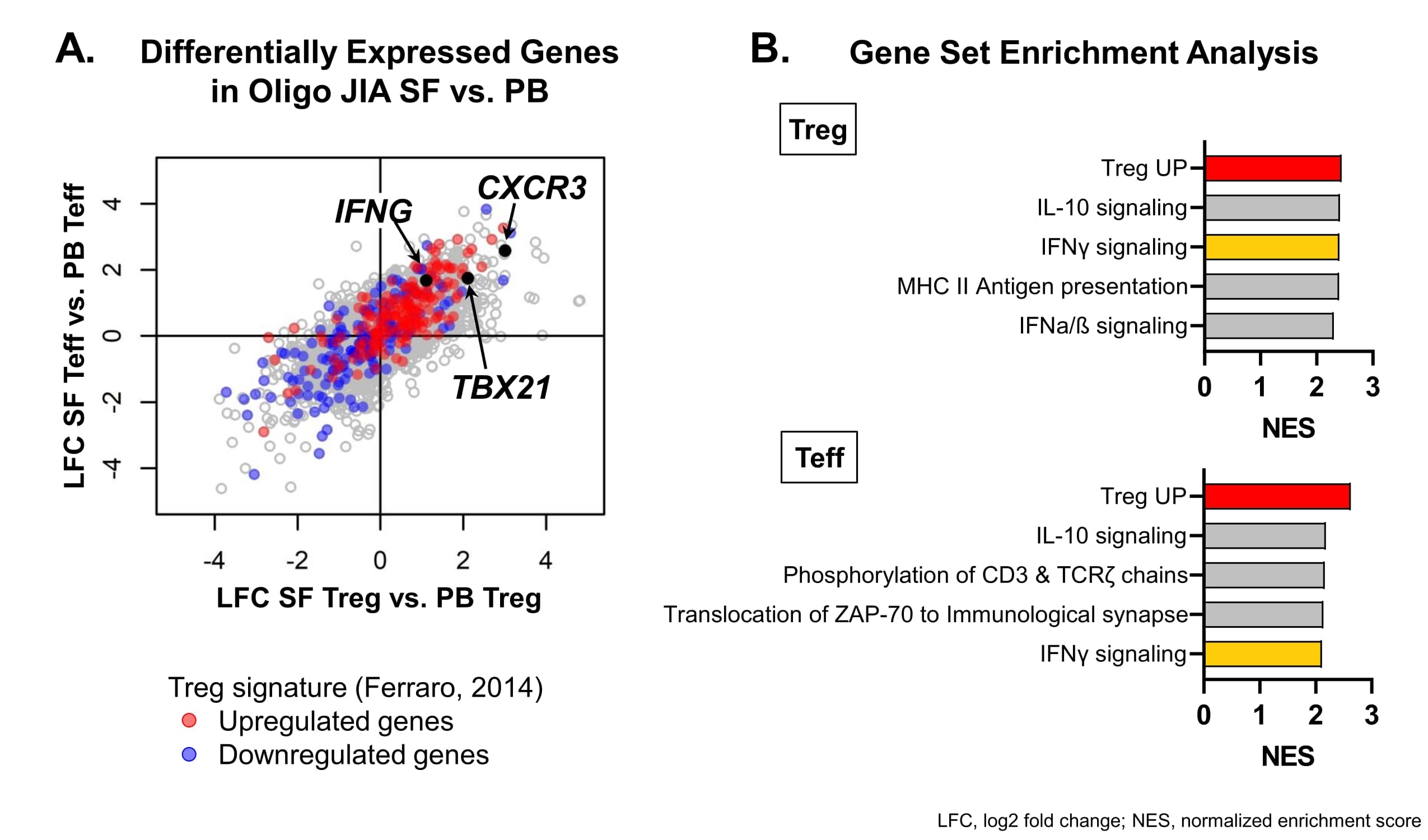 Oligo JIA SF Treg and SF Teff show markers of a Th1 transcriptomic profile. A) Log2 fold change vs. Log2 fold change plot of the outcomes from the differential expression analysis of oligo JIA SF (n=14, including 7 new-onset and 7 persistent oligo JIA) versus PB (n = 22, including 8 oligo JIA and 14 HC) in Tregs and Teffs. Genes upregulated (red) and downregulated (blue) in the Treg signature described in Ferraro (2014) are highlighted. Th1-related genes (CXCR3, TBX21, IFNG) are labeled. B) Top enriched gene sets in oligo JIA SF Tregs vs. PB Tregs and oligo JIA SF Teffs vs. PB Teffs based on the normalized enrichment score (NES) from the gene set enrichment analysis against the Reactome Database (Immune system and Metabolism subsets) and the Treg signature (Ferraro, 2014).
Oligo JIA SF Treg and SF Teff show markers of a Th1 transcriptomic profile. A) Log2 fold change vs. Log2 fold change plot of the outcomes from the differential expression analysis of oligo JIA SF (n=14, including 7 new-onset and 7 persistent oligo JIA) versus PB (n = 22, including 8 oligo JIA and 14 HC) in Tregs and Teffs. Genes upregulated (red) and downregulated (blue) in the Treg signature described in Ferraro (2014) are highlighted. Th1-related genes (CXCR3, TBX21, IFNG) are labeled. B) Top enriched gene sets in oligo JIA SF Tregs vs. PB Tregs and oligo JIA SF Teffs vs. PB Teffs based on the normalized enrichment score (NES) from the gene set enrichment analysis against the Reactome Database (Immune system and Metabolism subsets) and the Treg signature (Ferraro, 2014).
To cite this abstract in AMA style:
Jule A, Hoyt K, Wei K, Case S, Chang M, Cohen E, Dedeoglu F, Hazen M, Hausmann J, Halyabar O, Janssen E, Lee P, Lo J, Lo M, Meidan E, Roberts J, Son M, Sundel R, Chatila T, Nigrovic P, Henderson L. Th1 Polarization Defines the T Cell Compartment in the Joints of Oligoarticular Juvenile Idiopathic Arthritis Patients [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/th1-polarization-defines-the-t-cell-compartment-in-the-joints-of-oligoarticular-juvenile-idiopathic-arthritis-patients/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/th1-polarization-defines-the-t-cell-compartment-in-the-joints-of-oligoarticular-juvenile-idiopathic-arthritis-patients/
