Session Information
Date: Tuesday, October 28, 2025
Title: (1855–1876) Systemic Sclerosis & Related Disorders – Basic Science Poster II
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Emerging evidence implicates mitochondrial dysfunction as a contributor to tissue fibrosis in systemic sclerosis (SSc) (1). Endothelial cells, which are a key player in vascular injury and immune activation, are increasingly recognized as one of the main drivers of both fibrotic and type I interferon responses in SSc tissues (2). Since increased TGF-β stimulation is a hallmark of SSc pathogenesis, we hypothesized that it drives mitochondrial stress and impairs mitophagy in SSc endothelial cells, leading to cytosolic mitochondrial DNA (mtDNA) accumulation and subsequent activation of the cGAS-STING pathway that ultimately promote disease progression.
Methods: Primary dermal microvascular endothelial cells (MVECs) and fibroblasts from healthy donors (HD) and SSc patients were treated with TGF-β (10 ng/mL), IL-4 (5 ng/mL, negative control) or CCCP (positive control for mitophagy). Mitochondrial fission and stress-related transcripts were assessed by RT-qPCR. Oxygen Consumption Rate (OCR) was measured via Seahorse assays. Mitochondrial morphology and content were analysed via Immunofluorescence using MitoTracker Deep Red. Immunoblots were used to evaluate cGAS-STING pathway activation (P-STING, P-IRF3). The presence of mtDNA release in the supernatant was assessed by qPCR. Conditioned media from stimulated cells were tested in THP-1 dual luciferase reporter cells for ISRE/IRF3-driven transcription.
Results: At steady-state, MVECs from SSc patients exhibited an elevated expression of mitochondrial fission genes and a reduction in mitochondrial content compared to HD MVEC, suggesting defective mitochondrial quality control in SSc MVECS. Fibroblasts and MVECs from HD exposed to TGF-β upregulated multiple mitochondrial fission and mitophagy-related genes (DRP1, RALA, SPIRE1, HDN1) after 24 hours. Long-term TGF-β exposure (7 days) in HD MVECs led to a 2.5-fold reduction in baseline and maximal OCR as assessed by Seahorse analysis. Concordantly, Seahorse assays revealed a marked decrease in basal and maximal respiration in SSc MVECs compared to HD MVECs at baseline. Short-term TGF-β treatment (6h) of MVECs induced cGAS-STING activation, as evidenced by increased P-STING and P-IRF3. Conditioned media from TGF-β-treated MVECs triggered time-dependent IRF3-driven luciferase activity in THP-1 reporter cells, correlating with duration of TGF-β exposure, suggesting the hypothesis of a paracrine transmission of mtDNA or other interferon stimulating mediators.
Conclusion: Long-term TGF-β exposure induces mitochondrial fragmentation, stress, and dysfunctional mitophagy in HD MVECs. This leads to cytosolic accumulation and extracellular release of mtDNA and induces a low-respiration phenotype similar to SSc primary MVECs. This process triggers intracellular and paracrine activation of the cGAS-STING axis. Our findings uncover a novel mechanistic link between TGF-β signalling, mitochondrial dysfunction, and innate immune activation in SSc endothelium, identifying mitochondrial quality control as a potential therapeutic target
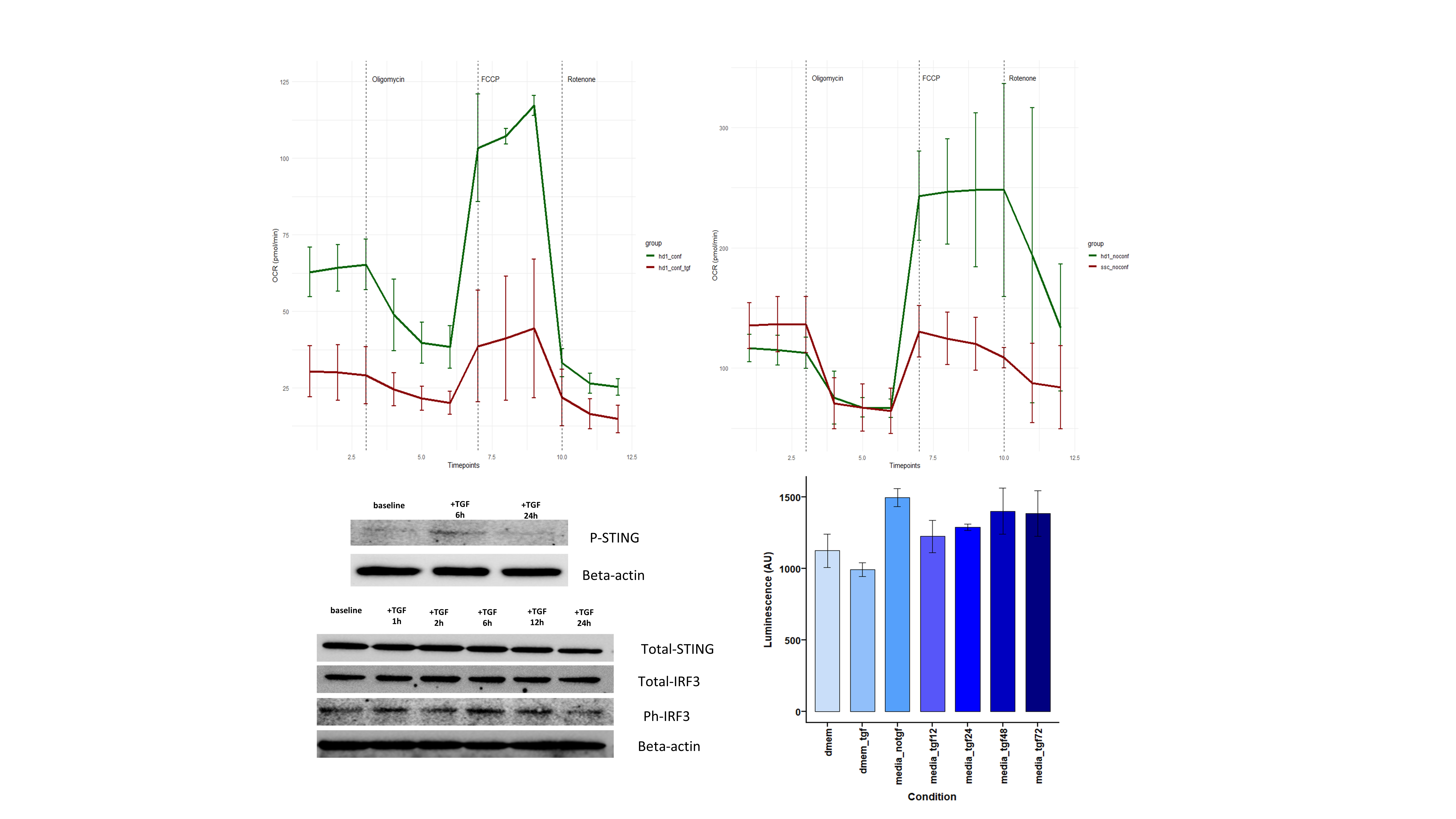 Figure 1. Mitochondrial dysfunction and STING pathway activation in endothelial cells treated with TGF-β. Top: Seahorse OCR traces showing reduced mitochondrial respiration in TGF-β treated endothelial cells (left: healthy untreated vs healthy treated with TGF; right: healthy untreated vs Systemic Sclerosis untreated). Dotted lines indicate the addition of oligomycin, FCCP, and rotenone. Data are presented as mean ± SEM. Bottom left: Western blot showing P-STING, total STING, total IRF3, and phosphorylated IRF3 (Ph-IRF3) in endothelial cells at baseline and after TGF-β stimulation. Beta-actin was used as a loading control. Bottom right: Luminescence assay quantifying cGAS-STING pathway activation at different time points following TGF-β treatment, confirming sustained activation at 12-72h.
Figure 1. Mitochondrial dysfunction and STING pathway activation in endothelial cells treated with TGF-β. Top: Seahorse OCR traces showing reduced mitochondrial respiration in TGF-β treated endothelial cells (left: healthy untreated vs healthy treated with TGF; right: healthy untreated vs Systemic Sclerosis untreated). Dotted lines indicate the addition of oligomycin, FCCP, and rotenone. Data are presented as mean ± SEM. Bottom left: Western blot showing P-STING, total STING, total IRF3, and phosphorylated IRF3 (Ph-IRF3) in endothelial cells at baseline and after TGF-β stimulation. Beta-actin was used as a loading control. Bottom right: Luminescence assay quantifying cGAS-STING pathway activation at different time points following TGF-β treatment, confirming sustained activation at 12-72h.
To cite this abstract in AMA style:
Di Donato S, Bordes C, Lalou C, Depaire a, Tchen J, Lhuissier C, Brisou d, Sisirak V, Garaude J, Wasson C, Ross R, Del Galdo F, Truchetet M. TGF-β-Driven Mitochondrial Stress Activates cGAS-STING Signalling via Impaired Mitophagy in Systemic Sclerosis Endothelial Cells [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/tgf-%ce%b2-driven-mitochondrial-stress-activates-cgas-sting-signalling-via-impaired-mitophagy-in-systemic-sclerosis-endothelial-cells/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tgf-%ce%b2-driven-mitochondrial-stress-activates-cgas-sting-signalling-via-impaired-mitophagy-in-systemic-sclerosis-endothelial-cells/
