Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Rheumatoid arthritis (RA) is a systemic autoimmune disease with synovial inflammation as the main pathological feature, and can eventually lead to irreversible joint or organ damage. Synovial macrophages (SMs) and synovial fibroblasts (SFs) play important roles in the occurrence and development of synovitis by secreting large amounts of cytokines and conducting crosstalk with various cells. However, understanding of the synovial inflammatory microenvironment and the mechanism for synovial cell activation remains limited, and there are no effective treatment methods that directly target synovial cells. The purpose of our study is to comprehensively and systematically explore the mechanism for Semaphorin 5A-mediated abnormal SF activation in RA.
Methods: Semaphorin 5A expression was assessed by ELISA, immunohistochemistry, immunofluorescence, quantitative polymerase chain reaction (qPCR), and western blotting in synovial fluid and synovial tissue samples from RA patients, osteoarthritis (OA) patients, and healthy controls. SF activation was evaluated by qPCR, CCK-8, wound healing, transwell, and flow cytometry assays. Transcriptome sequencing and protein array analyses were conducted to explore the regulatory pathway. Ferroptosis was observed by transmission electron microscopy, while lipid peroxidation was detected by MDA levels and C11-BODIPY 581/591 fluorescence intensity. Western blotting and transient small interfering RNA knockdown were performed to examine the regulatory role of Semaphorin 5A for SF activation in RA via PI3K/Akt/mTOR signaling.
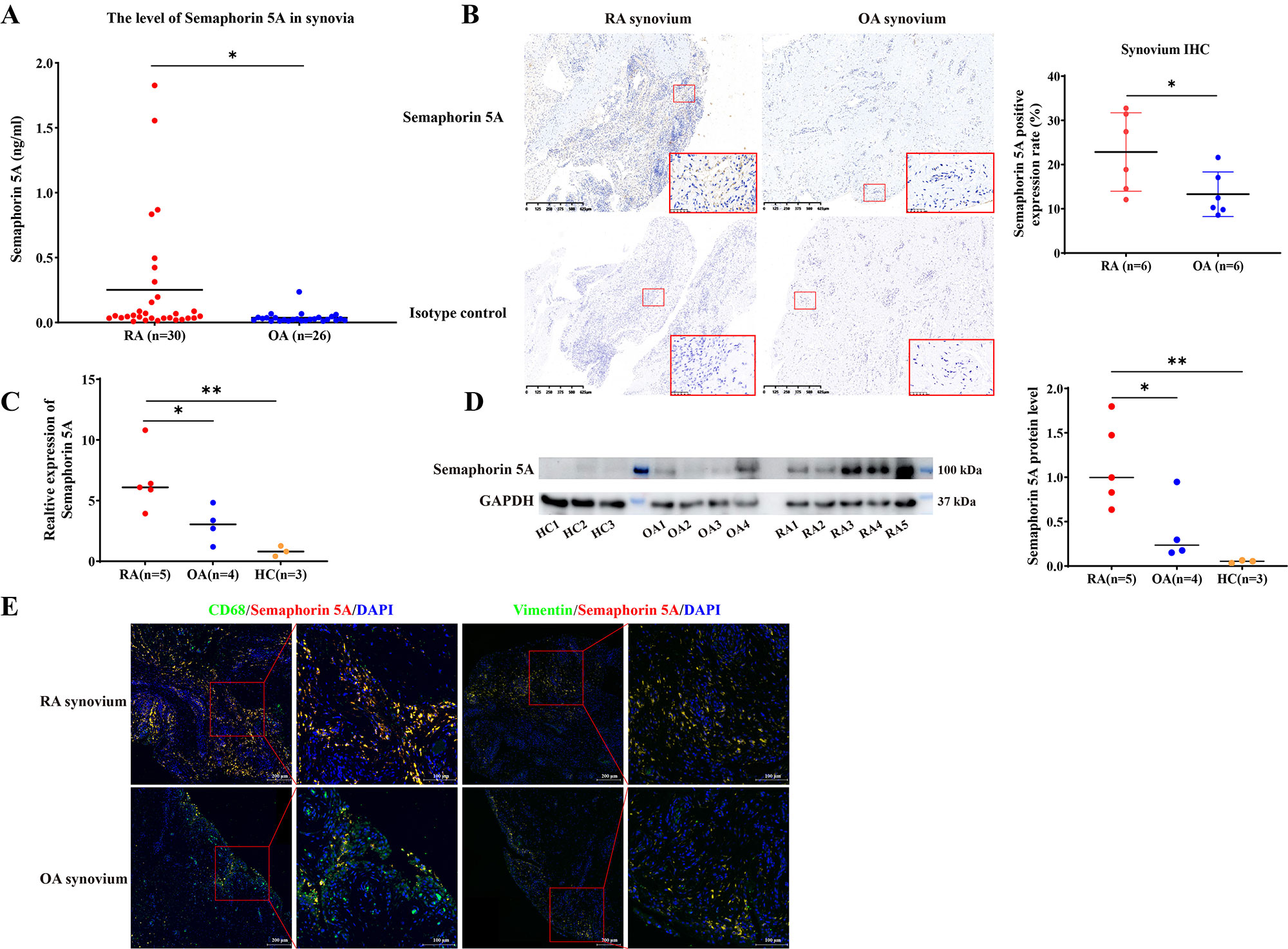
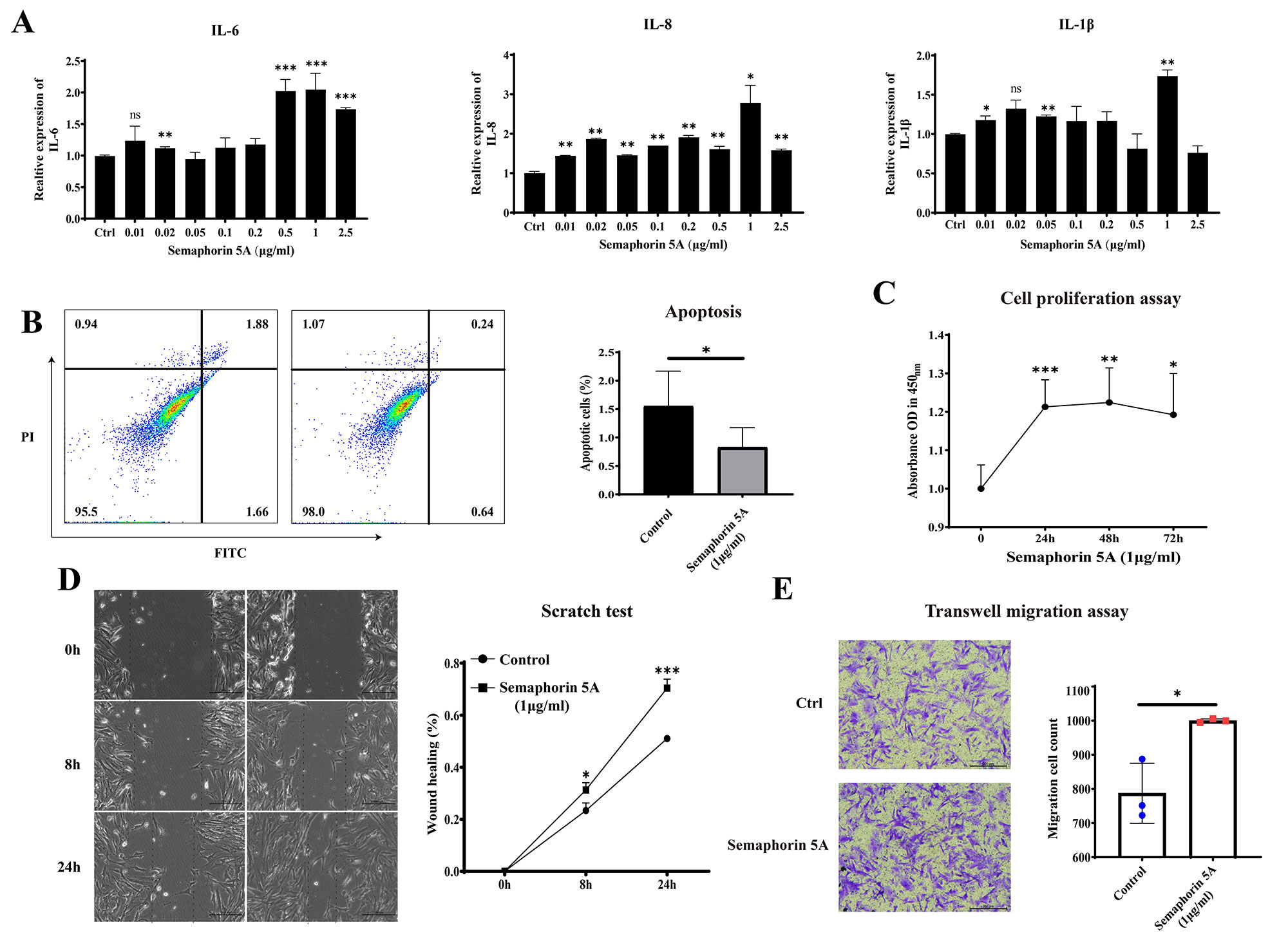
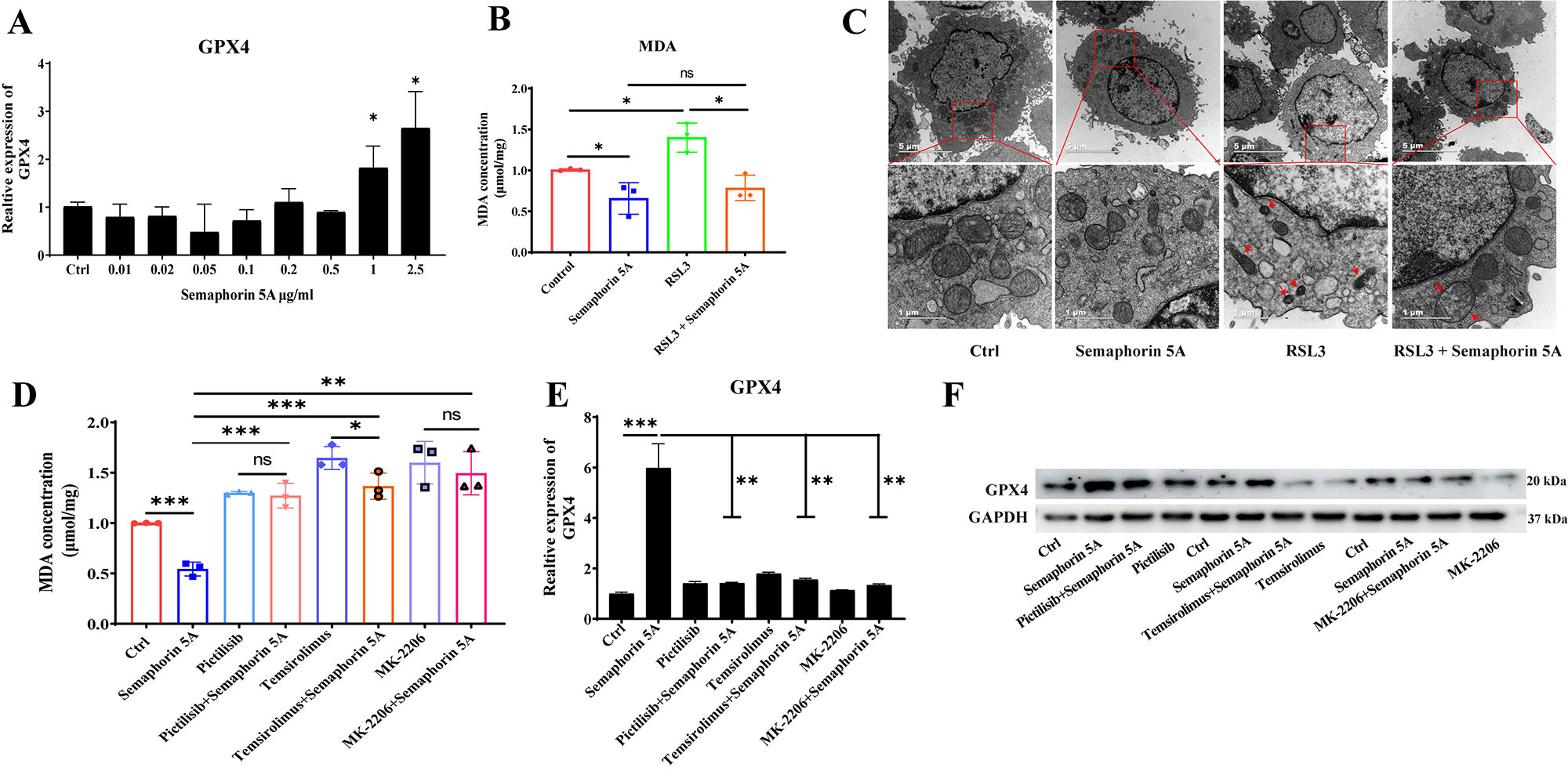
Results: Semaphorin 5A levels were significantly higher in synovial fluid and synovial tissue from RA patients compared with OA patients (Figure 1A-1D). The increased Semaphorin 5A in RA synovial fluid was derived from CD68+ synovial macrophages (Figure 1E), and the elevation led to increased binding between Semaphorin 5A and Plexin-A1/B3 receptors, thereby promoting cytokine secretion, proliferation, and migration, and decreasing apoptosis (Figure 2). Omics analyses revealed that Semaphorin 5A activated the PI3K/AKT/mTOR signaling pathway and inhibited ferroptosis. The stimulatory effect of Semaphorin 5A was abolished by pathway inhibitors. Morphologically, transmission electron microscopy results showed that Semaphorin 5A could significantly eliminate the mitochondrial diminution, membrane density increased and crest ruptured of SFs induced by ferroptosis inducer RSL3. Mechanistically, Semaphorin 5A enhanced GPX4 expression by activating the PI3K/AKT/mTOR signaling pathway, thus suppressing ferroptosis of RA SFs (Figure 3).
Conclusion: In conclusion, our study provided the first evidence that elevated Semaphorin 5A in RA synovial fluid promotes SF activation by suppressing ferroptosis through the PI3K/AKT/mTOR signaling pathway.
To cite this abstract in AMA style:
Cheng Q, chen M, Liu M, chen X, Wu H, Du Y. Semaphorin 5A Suppresses Ferroptosis Through Activation of PI3K-AKT-mTOR Signaling in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/semaphorin-5a-suppresses-ferroptosis-through-activation-of-pi3k-akt-mtor-signaling-in-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/semaphorin-5a-suppresses-ferroptosis-through-activation-of-pi3k-akt-mtor-signaling-in-rheumatoid-arthritis/