Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
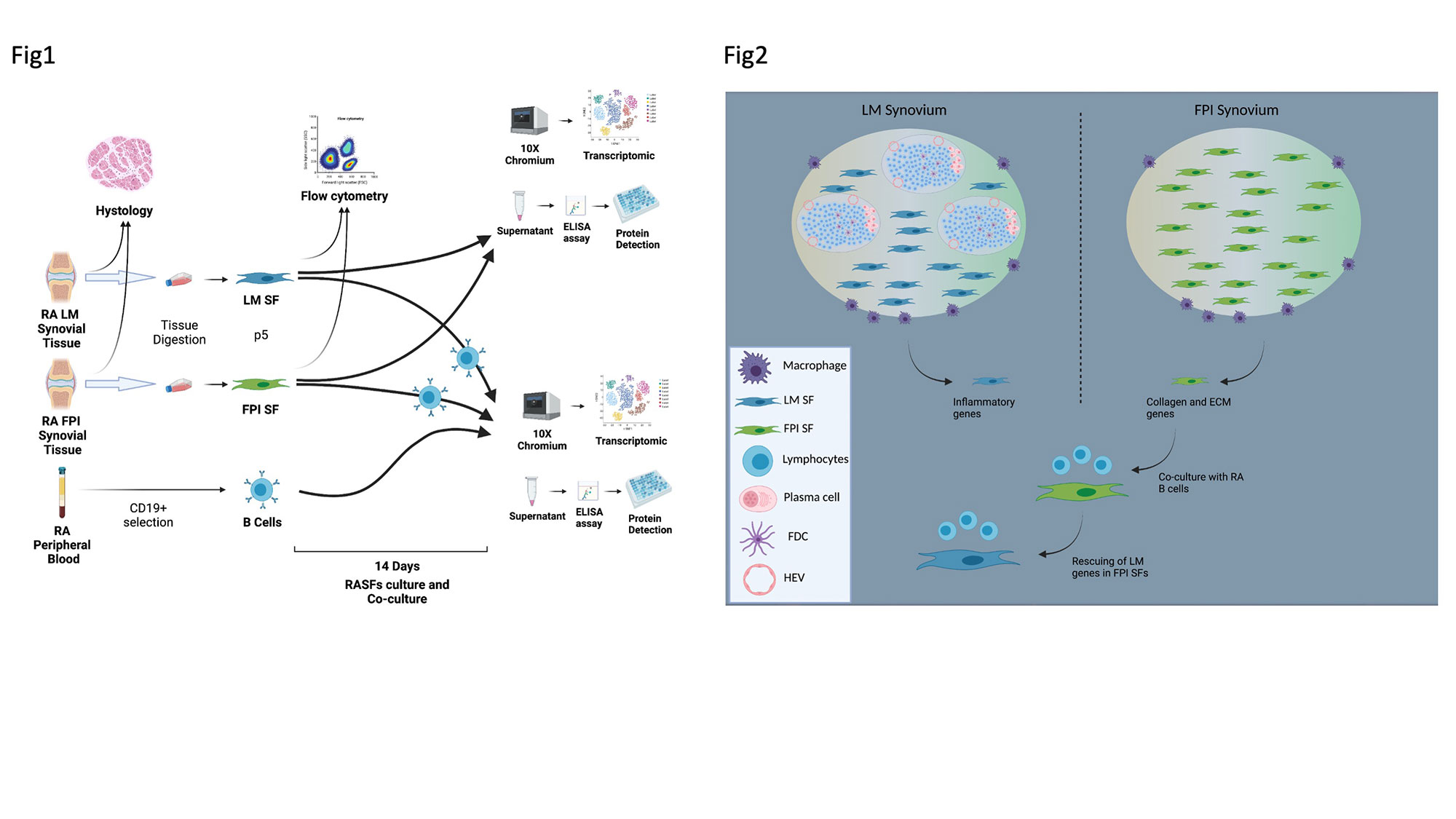
Background/Purpose: Despite advances in the treatment of Rheumatoid Arthritis (RA), synthetics and biologicals drugs are ineffective in ~40% of patients. The origin of this refractoriness is unclear, but several clues point at the synovial microenvironment (SE) and the relative cellular heterogeneity between patients. Indeed, the crosstalk between stromal and immune cells within the rheumatoid joints is critical for the perpetuation of chronic inflammation and autoimmunity. We previously described the existence of different RA endotypes such as the lympho-myeloid, LM, which is B-cell rich and the fibroid-paucimmune, FPI, which – on the other hand – is devoid of B-cells. In line with this, it is currently uncertain whether the different presence of cells subsets – particularly of synovial fibroblasts (SFs) – derived from different RA endotypes is driven by “imprinted” properties of the SFs or is shaped by the SE through interactions with infiltrating immune cells (or whether these differences account for any pharmacological resistance). We hypothesized that trough the comparison of freshly isolated SFs and primary established SFs cultures obtained from LM vs FPI RA synovial biopsies we would find the existence of both “imprinted” and “inducible” RASFs signatures.
Methods: We performed flowcytometry analysis and single cell RNA sequencing (sc-RNAseq) on SFs obtained from LM and FPI biopsies, in isolation or in co-culture with RA B cells. Next, supernatant has been screened trough Multiplex and ELISA. Furthermore, we compared our results to publicly available sc-RNAseq datasets on freshly isolated SFs and to our bulk-RNAseq data from clinical trials patients.
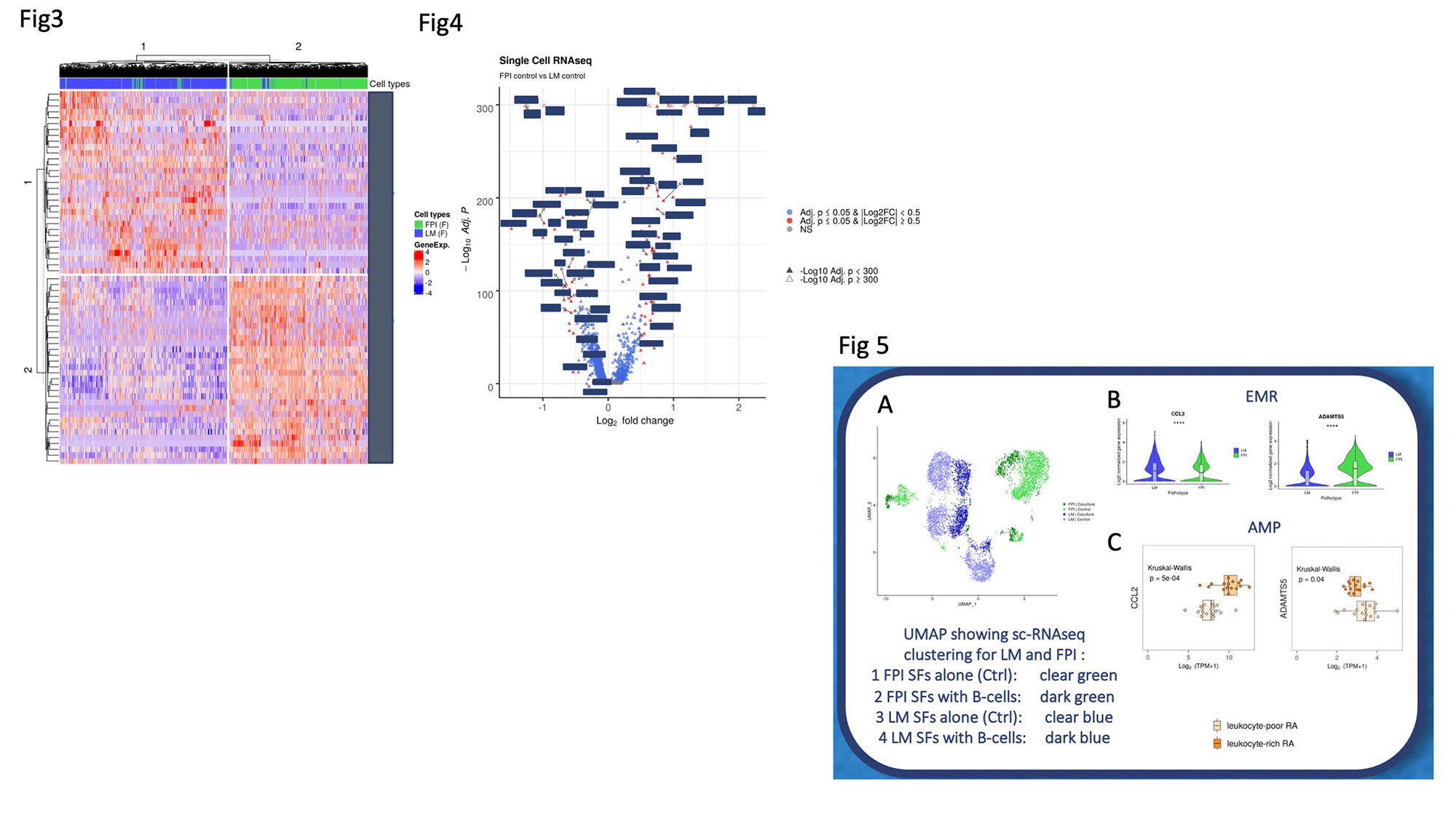
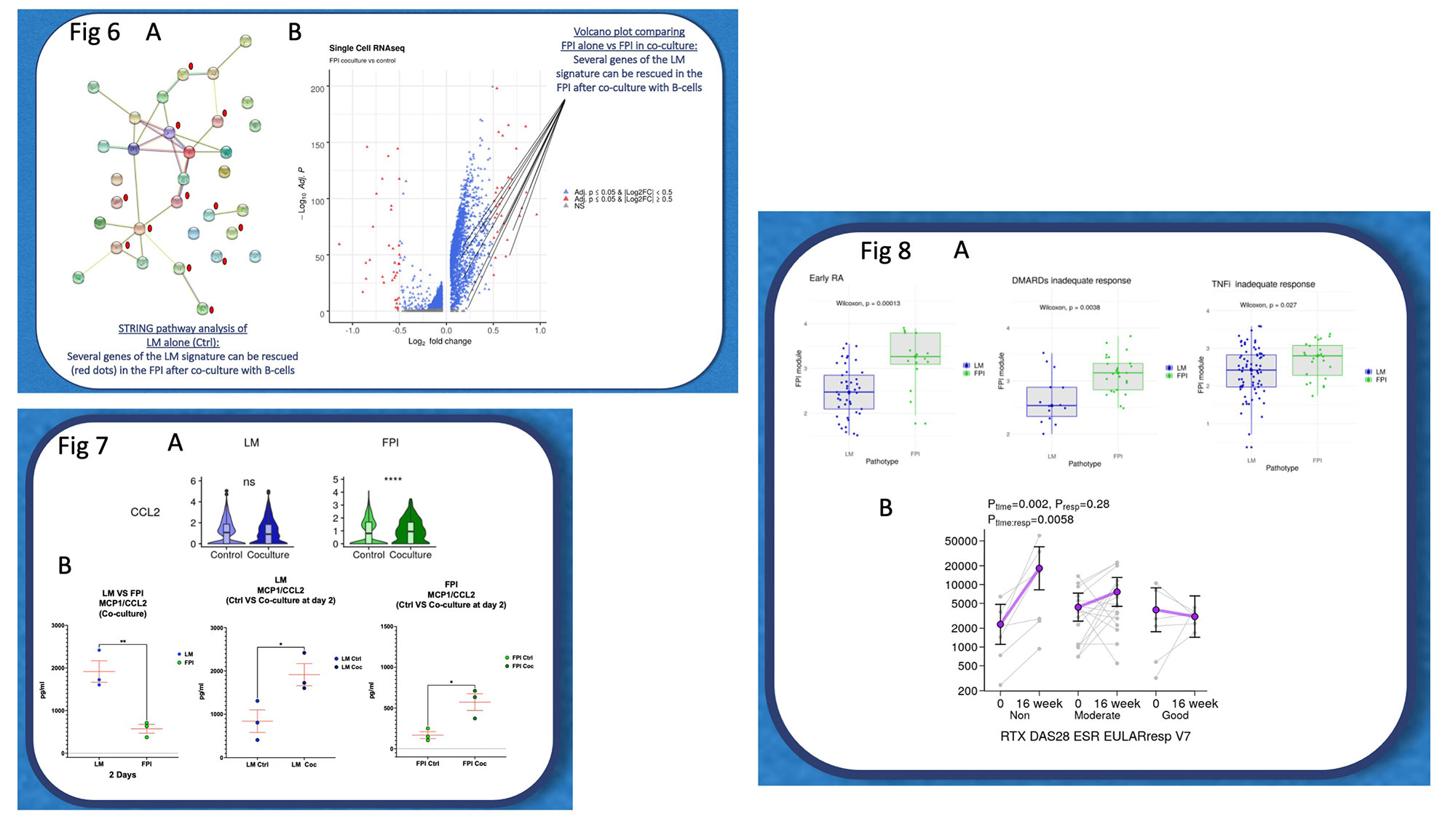
Results: Hierarchical clustering from sc-RNAseq transcriptional profiling of LM vs FPI RASF – after several cell passages – identified profoundly different gene signatures: whereby LM-RASF were characterised by genes involved in inflammation (IFI27/GAS6/CCL2/IL1R1), proteoglycan formation (LUM/DCN) and integrin binding (CD9/VCAM1/FBLN1), FPI-RASF were defined by genes such as ATF5, IGFBP5, CLEC3B as well as genes related to collagen biosynthesis (COL1A1/COL5A1). We speculate that cultured primary LM and FPI SFs identified footprints are probably reflecting their original tissue microenvironment. Also, these signatures can be partially tracked in larger clinical patient’s database of whole tissue bulk RNA-seq in both early arthritis and established RA. Finally, the modulation of FPI related genes following B-cell depletion identified poor responders to Rituximab in the R4RA randomised clinical trial. Strikingly, RA B-cells co-cultured with FPI-RASF over 14 days profoundly altered the FPI-RASF transcriptional profile including the ex-novo expression of genes typical of LM-RASF.
Conclusion: Our work shows that RASFs from different pathotypes bear different features, which are maintained in culture. Also, this imprinted memory can be dynamically modulated by the presence of B cells and can influence response/resistance to targeted biologic therapies. This work is particularly interesting in the context of refractory RA for a possible therapeutic exploitation.
Fig4: Volcano Plot showing comparison of LM RASFs (Left) and FPI RASFs (right) genes with magnitude of changes and significance (Nonsignificant genes: grey; Adjusted p-value: blue: adjusted p-value and log2 fold change: red, filled triangle: -log10 Adjusted p-value<300, empty triangles: -log10 Adjusted p-value>300).
Fig5: A: UMAP showing Control (Ctrl, alone cultured cells) and co-cultured SFs merged a re-clustered. B: Violin plot of CCL2 and ADAMTS5 expression between LM and FPI. Wilcoxon Test. C: Dot-plot on bulk RNAseq on freshly sorted SFs obtained from Accelerating Medicines Partnership (AMP, https://immunogenomics.io/ampra/ Defining Inflammatory Cell States in Rheumatoid Arthritis Joint Synovial Tissues by Integrating Single-cell Transcriptomics and Mass Cytometry, Zhang et al, Nature Immunology, 2019) comparing CCL2 andADAMTS5 expression on leukocyte-poor and rich RA ( AMP representation of our FPI and LM classification relatively). Values are expressed by fold change (Log2(TPM+1) transcript por Million). Kruskal Wallis.
Fig 7: A: Comparison LM RASFs (blue) and FPI RASFs (green) violin plots before and after B lymphocytes coculture, showing increase of CCL2 and rescue in FPI RASFs. B: MCP1/CCL2 concentration at day 2 in supernatants from Co-culture LM and FPI RASFs showing higher concentration in LM RASFs. Unpaired T test. Unpaired T test; MCP1/CCL2 concentration at day 2 in supernatants from Ctrl LM and co-cultured LM RASFs showing higher concentration in the latter. Unpaired T test; MCP1/CCL2 concentration at day 2 in supernatants from Ctrl FPI and co-cultured FPI RASFs showing higher concentration in the latter. Unpaired T test;
Fig 8: A: Summary box-plots on bulk RNA seq obtained from Early RA PEAC cohort, showing increased presence of FPI signature in the fibroid pathotype patients. Wilcoxon rank-sum test (also known as Manon-Whitney test); Summary box-plots on bulk RNA seq obtained from DMARDs inadequate response (PEAC cohort 6 months) showing increased presence of FPI signature in the fibroid pathotype patients. Wilcoxon rank-sum test (also known as Manon-Whitney) test); Summary box-plots on bulk RNA seq obtained from TNFi inadequate response (R4RA cohort), showing increased presence of FPI signature in the fibroid pathotype patients. Wilcoxon rank-sum test (also known as Manon-Whitney test). B: single FPI gene expression between baseline and 16 weeks in rituximab treated patients of R4RA showing increase of the gene in the non-responders regarding the RTX DAS28 ESR EULAR response.
To cite this abstract in AMA style:
Prediletto E, Cubuk C, Pontarini E, Rivellese F, Nerviani A, Lucchesi D, Caliste M, Corsiero E, Ahmed M, hands r, Lewis M, Pitzalis C, Bombardieri M. Rheumatoid Synovial Fibroblasts Display Imprinted Memory of Their Synovial Endotype Which Can Be Plastically Modulated by B-cells Crosstalk [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/rheumatoid-synovial-fibroblasts-display-imprinted-memory-of-their-synovial-endotype-which-can-be-plastically-modulated-by-b-cells-crosstalk/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rheumatoid-synovial-fibroblasts-display-imprinted-memory-of-their-synovial-endotype-which-can-be-plastically-modulated-by-b-cells-crosstalk/