Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Favoring apoptosis of neutrophils infiltrating the joints of patients suffering from rheumatoid arthritis (RA) and perpetuating inflammation has recently been discussed as an alternative therapeutic strategy. We therefore wanted to identify the dysregulated molecular pathways of cell death in neutrophils of patients with RA.
Methods: We performed a proteomic analysis of the cytosolic proteins of neutrophils isolated from the blood of 3 healthy donors, 3 RA patients in remission and from the synovial fluid of 4 RA patients with a disease flare. Functional analysis was generated through the use of the software Ingenuity Pathway Analysis (IPA). Results were then confirmed by western blot analysis.
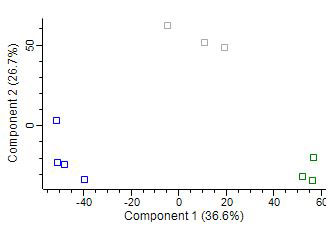
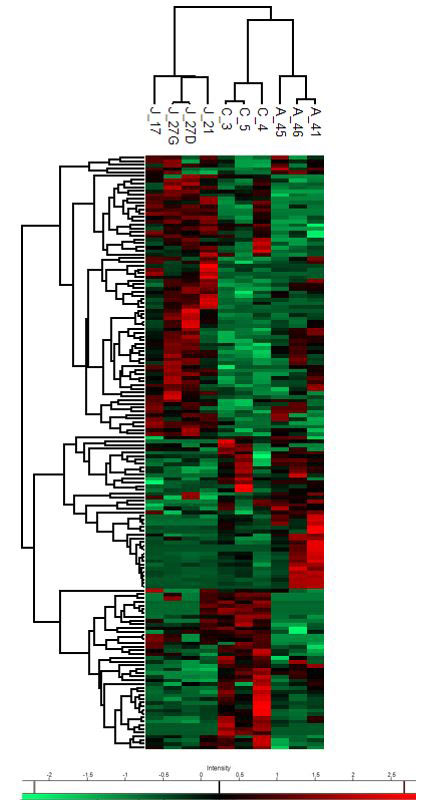
Results: Our proteomic analysis identified 2232 quantified proteins overall, out of which 64 were differentially expressed between RA blood and joint neutrophils. 43 were differentially expressed between RA blood and control blood and 83 were differentially expressed between RA joint and control blood neutrophils. Based on the differentially expressed proteins, IPA suggested a dysregulation of the death pathways in neutrophils isolated from blood and joint of RA patients. Most importantly, ferroptosis was one of the main signaling pathways modified in joint neutrophils of RA patients compared to blood neutrophils of healthy controls. Further, the pathway of pyroptosis was suggested to be disturbed in joint neutrophils compared to both blood neutrophils of RA patients as well as healthy controls. We identified seven proteins involved in ferroptosis that were differentially expressed between the three groups: arachidonate 15-lipoxygenase (ALOX15), ferritin heavy chain (FTH1), ADP-ribosylation factor 1, 4, 5 (ARF1, ARF4, ARF5), histone macro-H2A (MACROH2A1), cysteine-tRNA ligase (CARS1). Preliminary results of our western blot analysis confirmed the different expression of two proteins involved in ferroptosis (FTH1 and ALOX15).
Conclusion: We herein uncover that ferroptosis-associated proteins are dysregulated in neutrophils from patients with RA. Our results suggest that this pathway could be a future track of research for novel treatment strategy to achieve a short-term remission substituting corticosteroids, thus reducing their side effects.
C = blood neutrophils of healthy controls – A = blood neutrophils of RA patients – J = joint neutrophils of RA patients.
Color intensity indicates expression levels. The heatmap is coded red for increasing and green for decreasing. The colored bar on the bottom indicates how the heat map colors are related to the standard score (z-score).
To cite this abstract in AMA style:
Amsler J, Beaudouin C, LeGall M, Falgarone G, Rannou F, Tourneur L, Witko-Sarsat V. Proteomics Reveals Dysregulated Molecular Mechanisms of Cell Death in Neutrophils in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/proteomics-reveals-dysregulated-molecular-mechanisms-of-cell-death-in-neutrophils-in-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/proteomics-reveals-dysregulated-molecular-mechanisms-of-cell-death-in-neutrophils-in-rheumatoid-arthritis/