Session Information
Date: Saturday, November 6, 2021
Title: Abstracts: Systemic Sclerosis & Related Disorders – Clinical (0496–0501)
Session Type: Abstract Session
Session Time: 4:15PM-4:30PM
Background/Purpose: The Assessment of Systemic sclerosis-associated RAynaud’s Phenomenon (ASRAP) questionnaire is a novel patient-reported outcome instrument devised to assess the severity and impact of SSc-RP. The ASRAP items are grounded in the patient experience of SSc-RP. We report the development and subsequent assessment of internal reliability and preliminary construct validity testing of long and short-form versions of the ASRAP questionnaire.
Methods: The international multicentre ASRAP validation study enrolled English speaking SSc-RP patients from UK and US scleroderma centres during 2 consecutive winters 2019-2021. All participants completed a 39-item ASRAP questionnaire. Item reduction, scale scoring and derivation of a short-form ASRAP were based upon item response theory (IRT) assumptions and calibrated using the graded response model. The final 27-item long-form scores were correlated with a 10-item short form scores using Pearson’s correlation. Once scales were devised, preliminary assessment of construct validity was determined by Pearson’s correlation between long and short-form ASRAP scores and a range of concurrent disease specific and legacy instruments capturing SSc-RP patient measures of disease activity/impact and relevant domains including cold sensitivity, pain, function and patient global health.
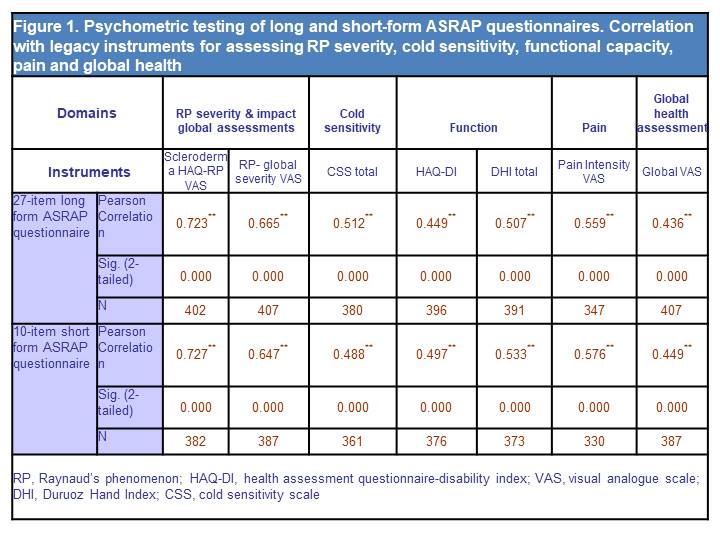
Results: A total of 438 SSc patients were enrolled at UK (n=238) and US (n=200) sites. A data driven approach with expert opinion was used to devise a 27-item long-form and 10-item short-form. Internal reliability between long and short form ASRAP questionnaires was excellent with Pearson correlation coefficient values of 0.976 (p< 0.0001). Evidence of construct validity of the long and short form ASRAP was indicated by strong agreement with existing global assessments of RP severity and impact (Scleroderma Health Assessment Questionnaire RP VAS and Patient RP global VAS (Figure 1). The short and long-form ASRAP questionnaire also had statistically significant correlations (in anticipated direction) with legacy instruments for assessing disability, hand function, pain and global health assessment (Figure 1).
Conclusion: Our preliminary analysis from the ASRAP validation study indicates strong internal reliability between long and putative short form ASRAP questionnaires. Cross-sectional psychometric analyses have indicated encouraging agreement with both global assessments of RP impact/severity and legacy instruments for assessing other relevant aspects of health pertinent to SSc. Further work to assess longitudinal reliability and sensitivity of change of the ASRAP questionnaire is planned.
To cite this abstract in AMA style:
Pauling J, Yu L, Denton C, Frech T, Herrick A, Hummers L, Saketkoo L, Shah A, Khanna D, Domsic R. Preliminary Assessment of Internal Reliability and Construct Validity of Long and Short-form Assessment of Systemic Sclerosis-associated RAynaud’s Phenomenon (ASRAP) Questionnaires [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/preliminary-assessment-of-internal-reliability-and-construct-validity-of-long-and-short-form-assessment-of-systemic-sclerosis-associated-raynauds-phenomenon-asrap-questionnaires/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/preliminary-assessment-of-internal-reliability-and-construct-validity-of-long-and-short-form-assessment-of-systemic-sclerosis-associated-raynauds-phenomenon-asrap-questionnaires/