Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
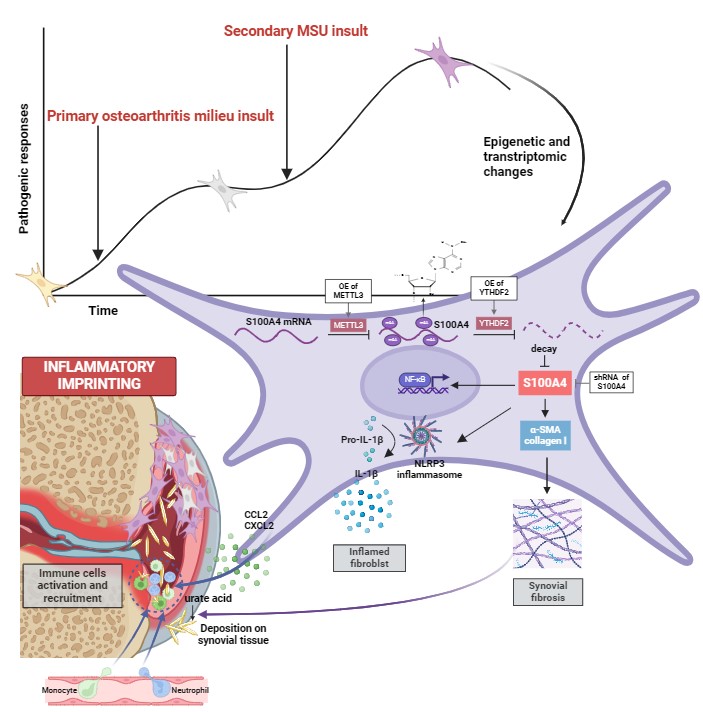
Background/Purpose: Patients with osteoarthritis (OA) face an elevated risk of future gouty arthritis (GA). To delineate the underlying cellular and molecular mechanisms of OA-driven gout exacerbation, we previously introduced the concept of “joint damage-related events” as a contributing factor to gout pathogenesis. However, it remains elusive how the holistic complex chronic inflammatory milieu of OA contributes to the exacerbation of gout. Inflammatory imprinting has recently been used to explain the occurrence of inflammatory comorbidities. In arthritis, synovial fibroblasts (SFs) are important contributors to the localized inflammatory imprinting pathology. Herein, we aimed to investigate whether OA-driven inflammatory imprinting of SFs can sustain arthritic traits and accelerate GA.
Methods: Firstly, we explored the phenotypes of patients-sourced osteoarthritic synovial fibroblasts stimulated with monosodium urate (MSU) crystals. Specifically, the inflammatory response of human osteoarthritic synovial fibroblasts stimulated with MSU (imprinted SFs) were analyzed using 2D and 3D cell culture; the interaction between imprinted SFs and macrophages, neutrophils were then analyzed in vitro co-culture systems and in vivo air pouch models; fibrotic characteristics of imprinted SFs were explored and their association with crystallization were also investigated in vitro crystal precipitation experiments. Subsequently, we identified the underlying mechanism using transcriptomic and epigenomic sequencing and bioinformatic analyses, and validated it by in vitro genetic targeting. Finally, we constructed GA post-OA rodent models to validate whether OA would drive inflammatory imprinting and exacerbate GA in vivo.
Results: We discovered that OA patients-derived SFs exhibited enhanced inflammatory and fibrotic functions in response to MSU stimulation, developing an inflammatory imprinting feature. Further transcriptomic and epigenomic analyses as well as genetic targeting revealed that this inflammatory imprinting relied on S100A4 upregulation via METTL3/YTHDF2-mediated m6A demethylation. Subsequently, we demonstrated that exposure to OA would induce synovial inflammatory imprinting and aggravate MSU-induced GA in rodent models, and it developed independently of synovial-resident macrophages. Finally, METTL3/S100A4 was synovium-targeted in rodent models, and it efficiently alleviated GA exacerbation by interrupting OA-induced synovial inflammatory imprinting.
Conclusion: Our study uncovered a role of SF-mediated epigenetic imprinting and residual memory in GA exacerbation post-OA, potentially informing therapeutic interventions and management of populations with OA who are at high risk of GA. Moreover, our findings also indicated that inflammatory memory in tissue-resident fibroblasts is an overlooked factor contributing to site-specific joint inflammatory comorbidities.
To cite this abstract in AMA style:
CHEN Z, Hua Y, Wang W. Osteoarthritis Drives Inflammatory Imprinting of Synovial Fibroblasts, Exacerbating Gouty Arthritis Through m6A Modification of S100A4 [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/osteoarthritis-drives-inflammatory-imprinting-of-synovial-fibroblasts-exacerbating-gouty-arthritis-through-m6a-modification-of-s100a4/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/osteoarthritis-drives-inflammatory-imprinting-of-synovial-fibroblasts-exacerbating-gouty-arthritis-through-m6a-modification-of-s100a4/