Session Information
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: RA symptoms are typically only assessed intermittently in clinical practice and clinical trials. Digital technologies (wearables, smartphones) can facilitate continuous remote monitoring of patients’ symptoms and response to treatment, though feasibility has not been evaluated. The aim of this study was to investigate if sensor data from the Apple Watch and iPhone, integrated with a mobile app, could provide clinically meaningful objective measures of function and collect PROs in patients with moderate-to-severe RA vs healthy controls (HCs).
Methods: Patients with moderate-to-severe RA (assessed by Routine Assessment of Patient Index Data 3) and HCs matched on age, gender, and race were recruited from Reliant Medical Group, MA, USA. Participants were given an Apple Watch and iPhone, and required to complete PROs either weekly, daily, or twice a day, and guided tests twice a day for 14 days. Multiple PROs, guided tests, and passive movement data were assessed in this study; however, this abstract focuses on stiffness, fatigue, joint pain map (JMAP)1, and the lie-to-stand (LTS) guided test. During the LTS test, sensors on the phone and watch collected objective movement data to measure the mean transition time from lying on a bed to standing up. Associations between PROs and guided test results were assessed using Pearson correlation and one-way analysis of variance (ANOVA).
Results: Of the 60 recruited participants, 2 patients with RA withdrew prior to study initiation, while 28 patients (moderate RA: n=13; severe RA: n=15), 28 matched HCs, and 2 unmatched HCs completed the study. Most participants were female (89%). Patients with RA had a significantly higher mean BMI vs HCs (31.4 kg/m2 vs 25.8 kg/m2; P=0.001).
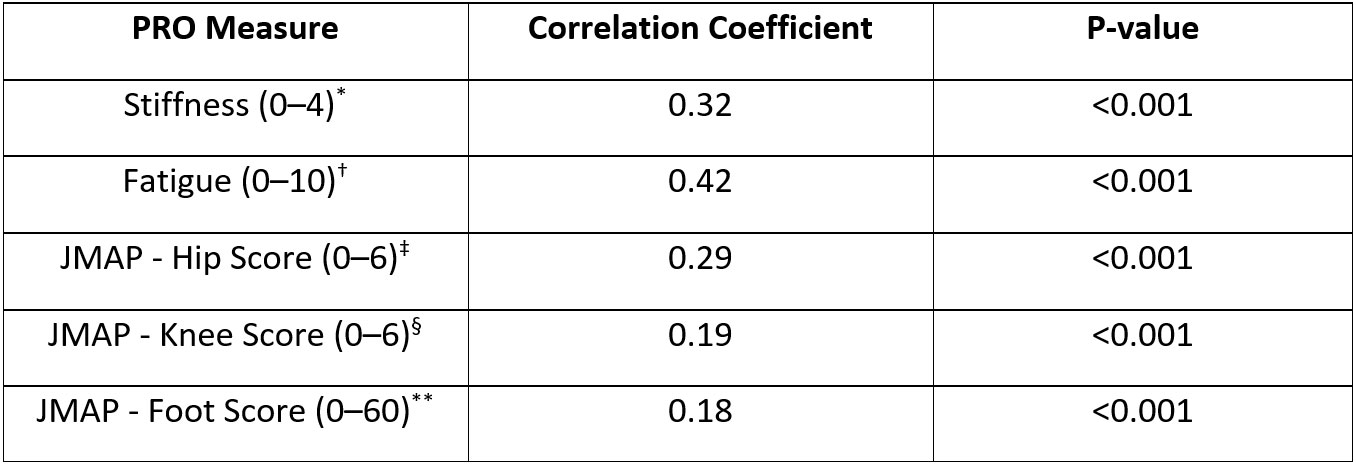
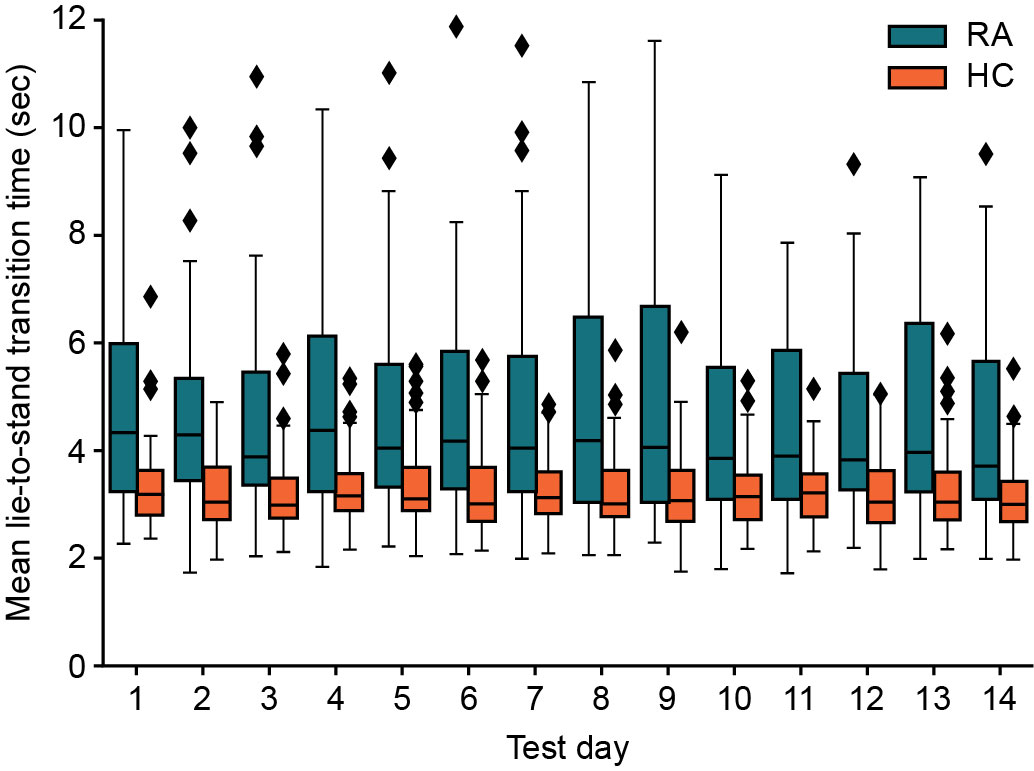
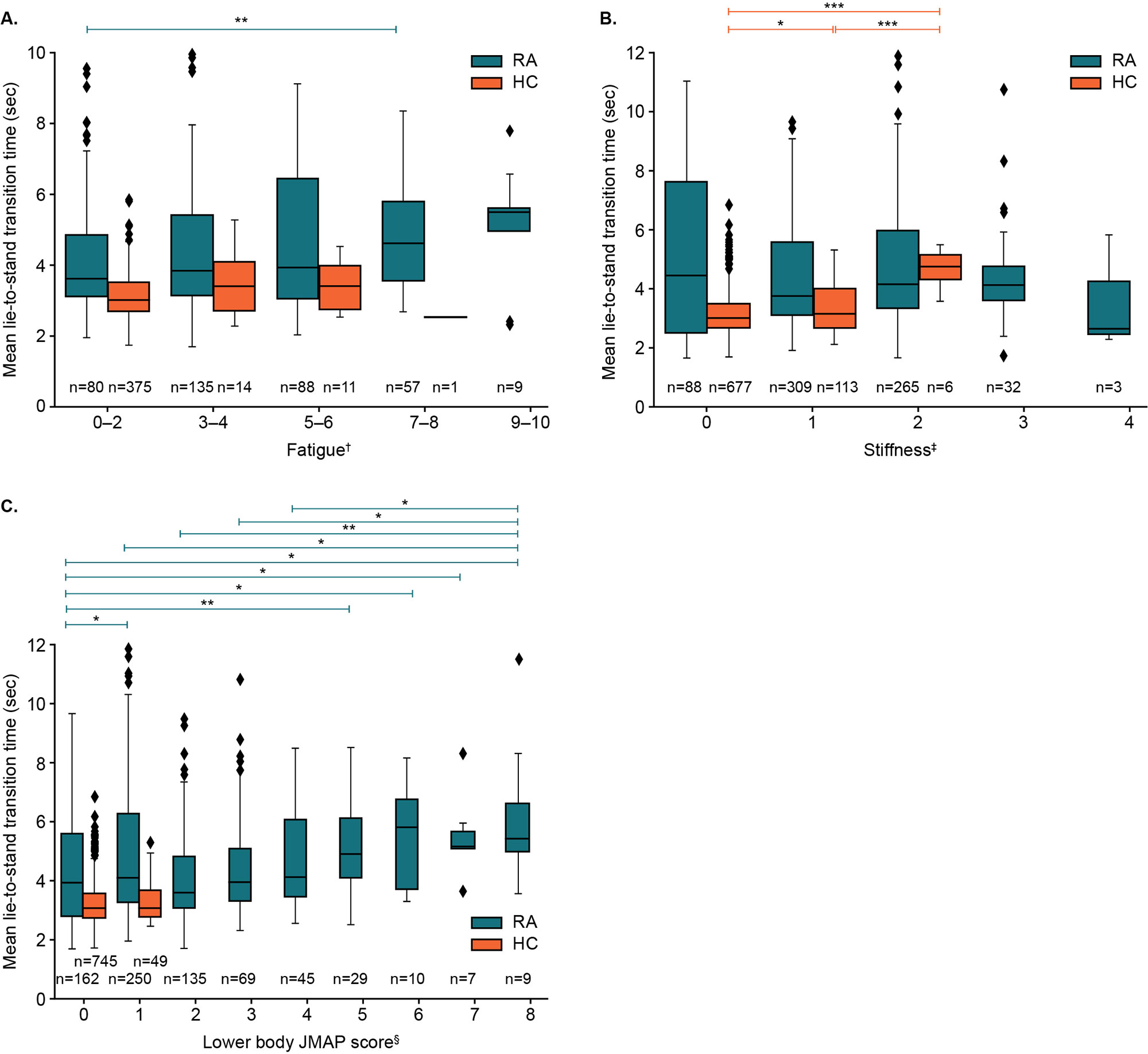
Participants had high compliance, completing >96% of PROs and guided tests and wearing the watch for >85% of the day. Objective measures of function were found to discriminate patient groups, e.g., patients with RA took a mean of 1.5 seconds longer to perform LTS transitions than HCs (P=0.001). A mixed effects linear model found no significant effect of study test day on the speed of the LTS test (Figure 1), indicating reproducibility of results over time. Although not statistically significant, mean transition time was longer in severe vs moderate RA (4.9 seconds vs 4.62 seconds; P=0.59). Longer LTS times significantly correlated with standard PRO scores, indicating good construct validity (Table); this was further supported by post hoc ANOVA tests showing that LTS times significantly separated the RA and HC cohorts, per standard PRO scores (Figure 2).
Conclusion: The LTS guided test was sensitive to differences in RA vs HC cohorts, and transition time correlated to symptom severity as measured by standard PROs. These results, and high compliance rates, demonstrate the feasibility of using smart devices integrated with a mobile app to collect meaningful patient-centric data in moderate-to-severe RA. Further studies are warranted to evaluate the potential of using this technology in a remote clinical trial setting.
GSK study (212295); medical writing support by Fishawack Indicia Ltd, funded by GSK.
References:
1. Hamy V, et al. Digit Biomark. 2020 Apr 30;4(1):26-43
Non-parametric Pearson correlation coefficients were used to examine the relationships between lie-to-stand transition times and various PROs scores.
*Patients with RA evaluated their morning and afternoon stiffness on a scale from 0 (no stiffness) to 4 (very severe). Stiffness was evaluated using the RA symptoms and pain questionnaire on days 1, 7, and 14, and by morning/afternoon stiffness assessments on the remaining study days; †Patients with RA evaluated their fatigue once a day on a scale from 0 (no fatigue) to 10 (as bad as you can imagine); ‡Patients with RA evaluated their left and right hip pain on a scale from 0 (no pain) to 3 (very severe). These scores were summed for a combined hip score; §Patients with RA evaluated their left and right knee pain on a scale from 0 (no pain) to 3 (very severe). These scores were summed for a combined knee score; **Patients with RA evaluated their pain in each of the 20 joints in their left and right feet on a scale from 0 (no pain) to 3 (very severe). These scores were summed for a combined foot score.
JMAP, joint pain map; PRO, patient-reported outcome; RA, rheumatoid arthritis.
A univariate mixed effects linear model with study day as the fixed effect and individual differences as the random effect. Box and whisker plots: Line in box represents mean; upper and lower limits of the box represent 1st and 3rd quartiles; upper and lower whiskers represent min and max. Diamond symbols represent outlier data points.
HC, healthy control; RA, rheumatoid arthritis.
One-way ANOVA post hoc analyses using Mann-Whitney U-tests were used to compare RA and HC cohorts. *P<0.05. **P<0.01, ***P<0.001
†Fatigue was assessed through a single-item question asking participants to rate their worst level of fatigue over the past 24 hours on a 10-point scale, ranging from “no fatigue” (0) to “as bad as you can imagine” (10); ‡Stiffness was assessed using a single-item question on the severity of morning stiffness experienced by the participant on a given day, with 5 response options provided, ranging from no stiffness (0) to very severe (4). §Lower body JMAP score was calculated by summing the scores for each hip, knee, and ankle joint (left and right; total=6), plus the average score of all foot joints (10 joints per foot) and dividing by 2. Score could range from a minimum of 0 to a maximum of 12 (maximum reached in study was 8).
ANOVA, analysis of variance; HC, health control; RA, rheumatoid arthritis.
To cite this abstract in AMA style:
Hamy V, Llop C, Yee C, Garcia-Gancedo L, Maxwell A, Chen W, Tomlinson R, Bobbili P, Bendelac J, Landry J, DerSarkissian M, Yenikomshian M, Mody E, Duh M, Williams R. Novel Digital Technologies for the Assessment of Objective Measures and PROs in Patients with Rheumatoid Arthritis: A Pilot Study Using Smart Devices and a Bespoke Mobile App [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/novel-digital-technologies-for-the-assessment-of-objective-measures-and-pros-in-patients-with-rheumatoid-arthritis-a-pilot-study-using-smart-devices-and-a-bespoke-mobile-app/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/novel-digital-technologies-for-the-assessment-of-objective-measures-and-pros-in-patients-with-rheumatoid-arthritis-a-pilot-study-using-smart-devices-and-a-bespoke-mobile-app/