Session Information
Date: Sunday, November 12, 2023
Title: (0066–0095) T Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Sustained chronic inflammation in the spine and of the sacroiliac joints is a key feature in Ankylosing Spondylitis (AS). A central role of CTL involvement in AS inflammation is supported by recent discoveries of chronically activated CTLs with distinctive cytotoxic and integrin expression profiles enriched at sites of inflammation. Yet, the immunophenotype of the CTL compartment which may perpetuate AS inflammation has not been fully defined. The microenvironment that is conducive to AS chronic inflammation is reminiscent to settings found in chronic viral infection and cancer. Here, we seek to unravel the complex heterogeneity of CTLs in AS patients to determine whether CTLs in AS are truly immunologically exhausted. We hypothesize that a loss of CTL immune homeostasis and evasion of T cell exhaustion contribute to autoinflammation in AS.
Methods: 8 pairs of PBMCs and SFMCs from patients with active (mean BASDAI=8) Axial Spondyloarthritis meeting the ASAS classification criteria were analysed. Additionally, age and sex-matched healthy control PBMCs (n=18) were analysed. We applied multi-omics single-cell immune profiling strategies to delineate the complex heterogeneity of CTLs in AS. Mass cytometry (CyTOF) of PBMCs and SFMCs was used to simultaneously measure protein expression of more than 30 surface and intracellular markers. Single-cell RNA sequencing (scRNAseq) and T cell receptor sequencing (scTCRseq) of FACS-sorted mature CTLs were used to immune profile AS CTL transcriptome and to perform clonal andTCR diversity analyses.
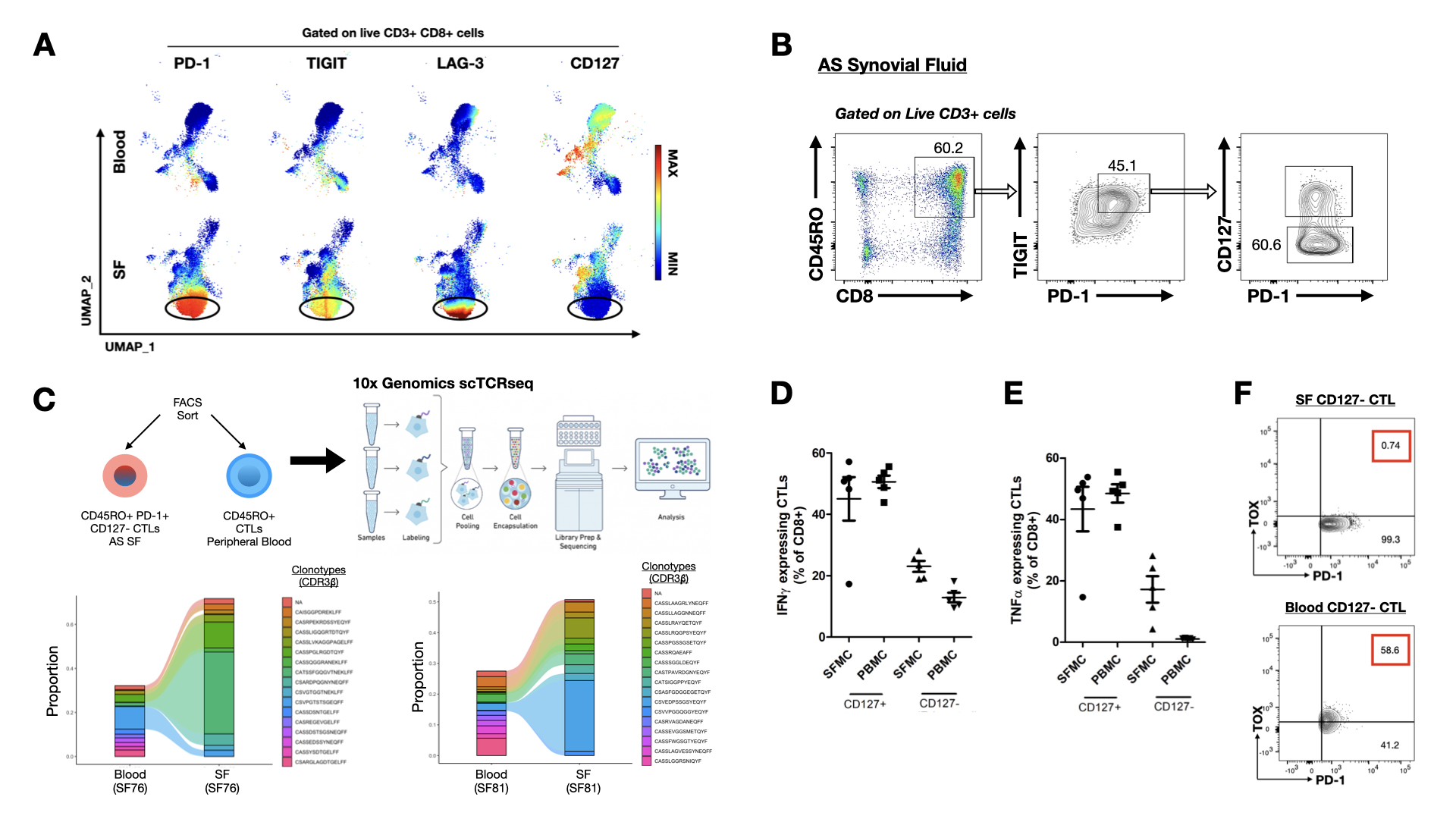
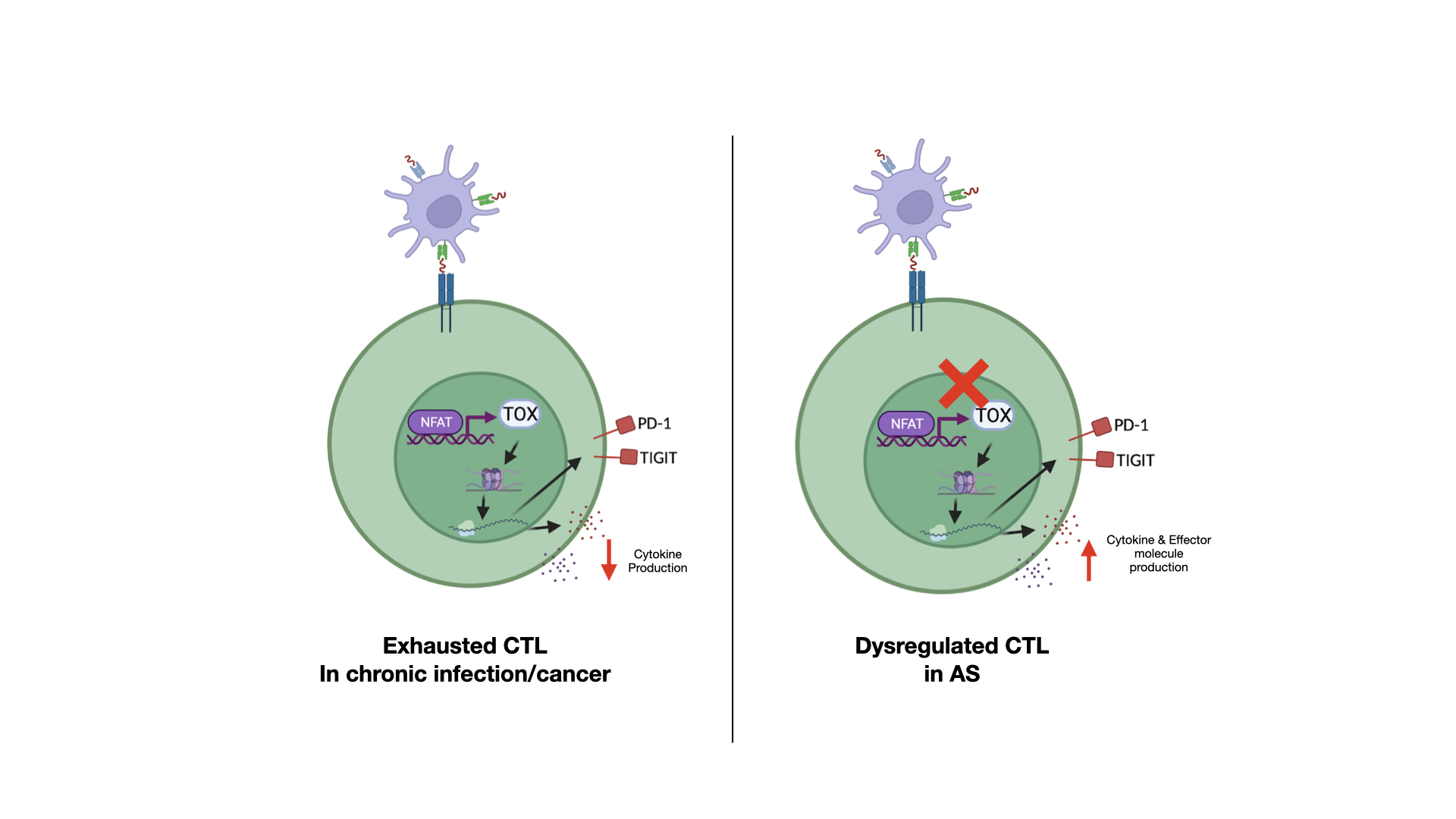
Results: Unsupervised clustering analysis of CyTOF data identified a subset of CTLs that co-express checkpoint receptors PD-1, TIGIT, and LAG-3 in SF CTLs of AS patients; while these markers are downregulated in the peripheral CTLs compared to healthy controls, implicating CTL dysregulation. This subset of SF CTLs downregulates CD127 expression, while retaining the capacity for CTL effector functions including the ability to express granzyme B, perforin and produce IFNγ and TNFαupon stimulation. Additionally, CD8+ T cell tissue residency markers (CD103, CD69, and CD49a) are highly upregulated in this subset of SF CTLs. scRNAseq of FACS-sorted CD45RO+ PD-1+ TIGIT+ CTLs revealed that transcripts that are normally expressed in canonically exhausted CTLs (e.g. CD244, HAVCR2, ENTPD1, NT5E, TOX) are downregulated. Differential gene analysis revealed that the top 5 genes (IFI6, MX1, ISG16, MT2A, IFITM1) upregulated in these cells are related to the interferon signalling pathway. Single cell TCR sequencing demonstrated that this CTL subset is clonally expanded at sites of inflammation. Intracellular flow cytometry confirmed that this CTL subset downregulated the master transcription factor regulating T cell exhaustion- TOX, suggesting that these CTLs are not truly immunologically exhausted.
Conclusion: Multi-omics immune profiling of CTLs from AS patients identified a subset of clonally expanded CTLs in SF that appear to resist immune exhaustion, while retaining classical cytotoxic capacities. This findings implicates that dysregulation of T cell exhaustion and homeostasis could potentially exacerbate AS autoinflammation.
To cite this abstract in AMA style:
Tang M, Qaiyum Z, Lim M, Inman R. Multi-omics Immune Profiling of Cytotoxic T Cells from Ankylosing Spondylitis Patients Identified a Subset of Clonally Exapnded CTLs That Evade Immune Exhaustion [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/multi-omics-immune-profiling-of-cytotoxic-t-cells-from-ankylosing-spondylitis-patients-identified-a-subset-of-clonally-exapnded-ctls-that-evade-immune-exhaustion/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/multi-omics-immune-profiling-of-cytotoxic-t-cells-from-ankylosing-spondylitis-patients-identified-a-subset-of-clonally-exapnded-ctls-that-evade-immune-exhaustion/