Session Information
Date: Saturday, November 6, 2021
Title: Systemic Sclerosis & Related Disorders – Clinical Poster I (0387–0413)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: The episodic and uniquely personalised nature of Raynaud’s phenomenon (RP) has led to reliance upon self-report to capture how patients ‘feel’ and ‘function’. Existing diary-based patient-reported outcome (PRO) instruments have not supported marketing authorisation of promising therapeutic interventions for RP in patients with systemic sclerosis (SSc). We report item development for the novel Assessment of Systemic sclerosis-associated RAynaud’s Phenomenon (ASRAP) questionnaire.
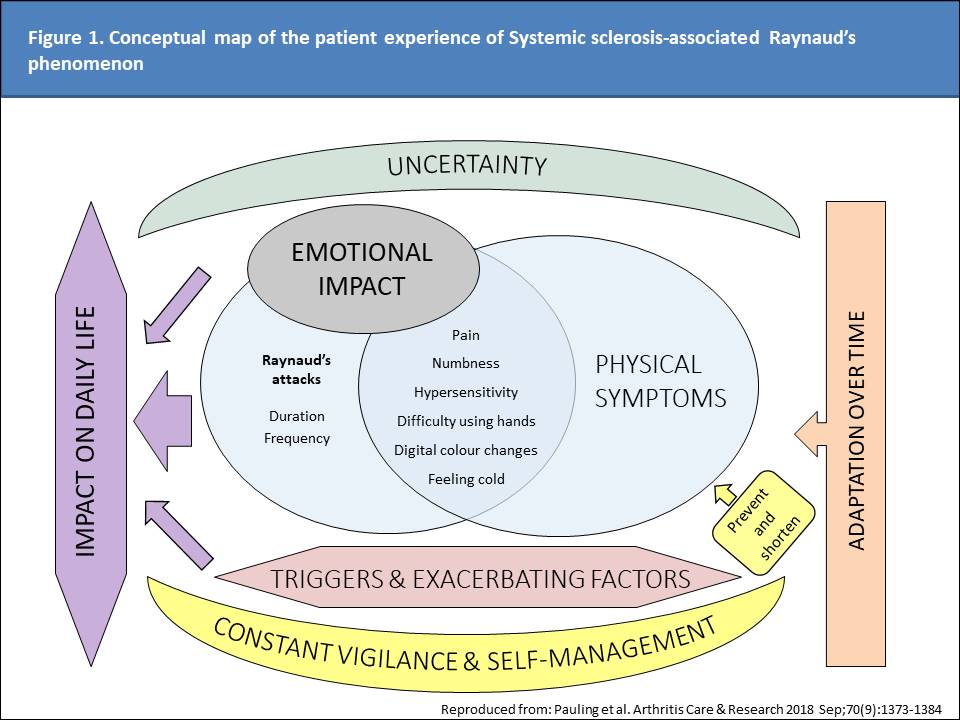
Methods: The conceptual framework for the ASRAP questionnaire was to devise a novel PRO instrument that captured the severity and impact of SSc-RP. A provisional item bank was developed with input from patient insight partners with candidate items grounded in the themes and sub-themes identified in an earlier international multicentre qualitative research study of SSc-RP (Figure 1). Iterative modification of the items was undertaken with input from 4 SSc experts and a patient research partner to ensure item wording, recall period and response options were simple, understandable, relevant to specific domain concepts and conformed to internationally agreed standards. Linguistic evaluation was assessed using the Simple Measure of Gobbledygook (SMOG) with modification to achieve a readability age of < 14 years. Cognitive de-briefing interviews were held with English-speaking patients at US and UK sites. An item tracking matrix was devised to document modifications throughout the item development process.
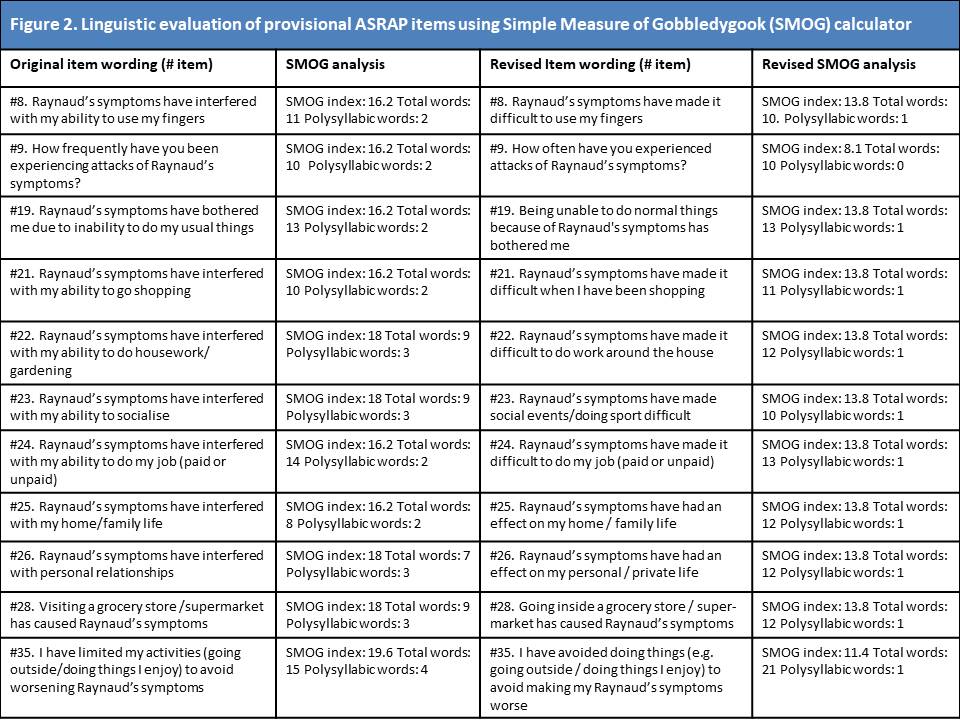
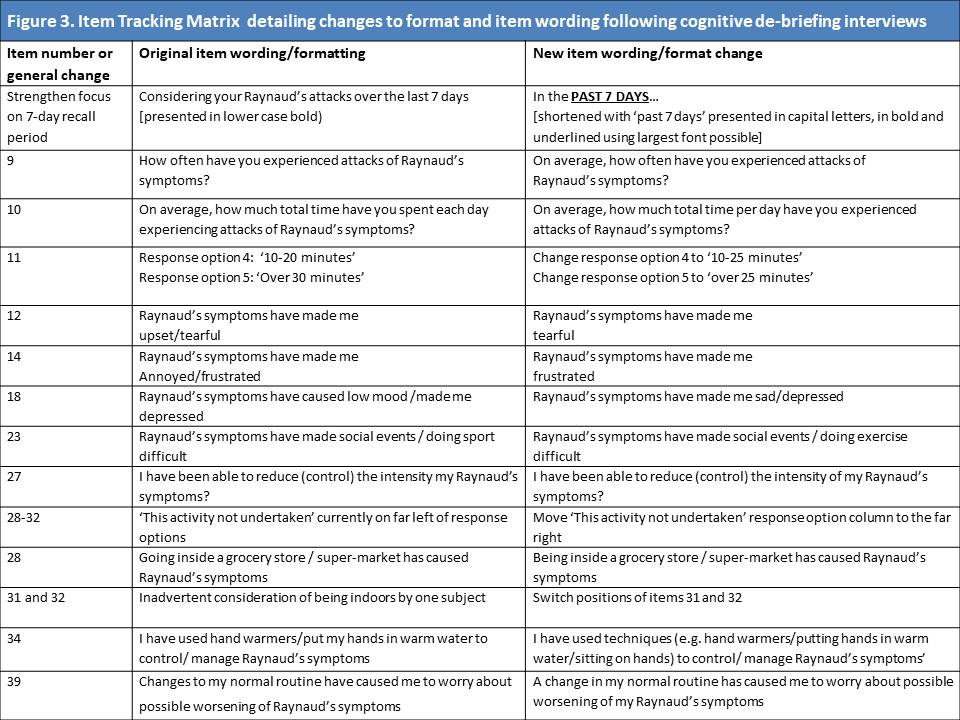
Results: A provisional item-bank of 37 candidate items was devised to capture the patient experience of SSc-RP with respect to physical symptoms (n=10), emotional distress (n=7), impact on daily life (n=6), exacerbating factors (n=6), self-management (n=4), adaptation (n=3) and uncertainty (n=2). An additional 2 items were proposed following expert review. Item recall period and response options were optimised for item response theory. Modification to 11 items to improve readability ensure a SMOG index < 14.0 for all items (Figure 2). Cognitive de-briefing interviews were held with 7 patients and led to modification to 11 items, alongside changes to format and structure of the ASRAP questionnaire (Figure 3). No new items were proposed during cognitive de-briefing.
Conclusion: The ASRAP questionnaire item bank has been devised and tested with direct input from an international consortium of SSc experts and patients. The items have been tested to ensure they reflect the intended conceptual framework and fully capture the lived experience of SSc-RP in wording that is comprehensible, minimizes ambiguity and meets accepted criteria for optimal translatability into non-English languages. The provisional 39-item ASRAP questionnaire shall progress to formal validation.
To cite this abstract in AMA style:
Pauling J, Saketkoo L, Khanna D, Denton C, Frech T, Herrick A, Hummers L, Shah A, Domsic R. Item Development for the Assessment of Systemic Sclerosis-associated Raynaud’s Phenomenon (ASRAP) Questionnaire [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/item-development-for-the-assessment-of-systemic-sclerosis-associated-raynauds-phenomenon-asrap-questionnaire/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/item-development-for-the-assessment-of-systemic-sclerosis-associated-raynauds-phenomenon-asrap-questionnaire/