Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Activated macrophages (Mph) can be subdivided in at least 2 major subgroups according to their polarization into classical pro-(M1-Mph) or anti-inflammatory (M2-Mph) subtypes. Together with fibroblasts they are considered key mediators of tissue remodeling in chronic rheumatic diseases. Mph possess high plasticity and it is suggested that an imbalance of M1/M2 Mph polarization might contribute to aberrant activation of fibroblasts (Fib) during tissue remodeling and destruction, like in rheumatoid arthritis (RA). However, the causes of disrupted M1/M2 homeostasis are still not clear. We conducted a project in order to investigate the interactions between synovial fibroblasts and differentiating and polarized macrophages in vitro.
Methods: Macrophages were differentiated from purified monocytes out of peripheral blood from healthy donors and polarized into M1 and M2 Mph over several days. Expression of CD80 TNFa and IL-12 were considered representative for M1 and expression of CD163, CD206 and IL-10 for M2. Fib isolated from osteoarthritis (OA) patients at passage 4-8 were co-cultured with cell culture supernatant (SN) of polarized M1/M2-Mph. Similarly, Fb-SN was added to differentiating Mph. For some experiments Fib were pre-stimulated with TNFa/IL1b to induce a proinflammatory RA-like phenotype. Fib read-out included cytokine and MMP secretion and transcriptional expression of key markers COX-2, COL-1 and aSMA.
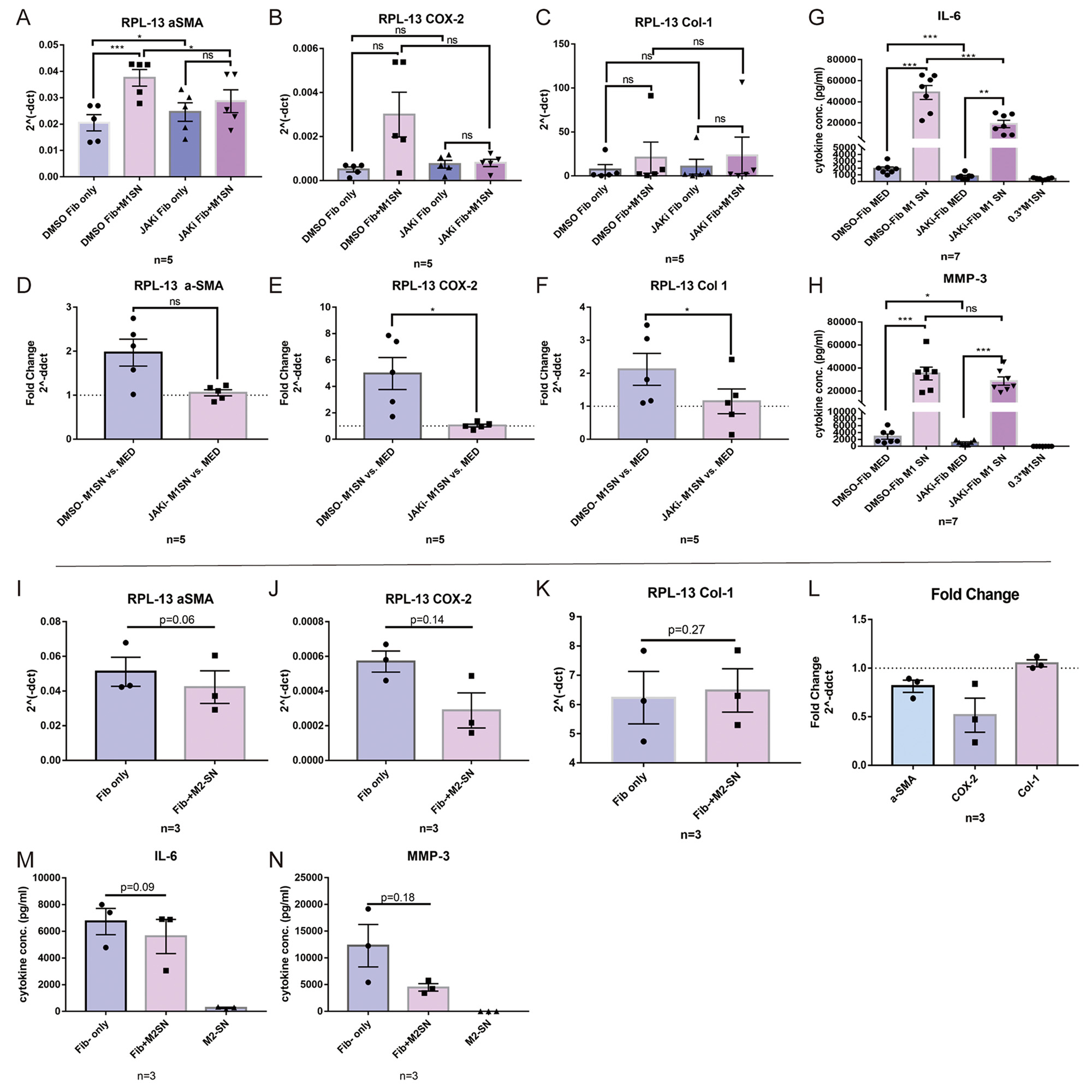
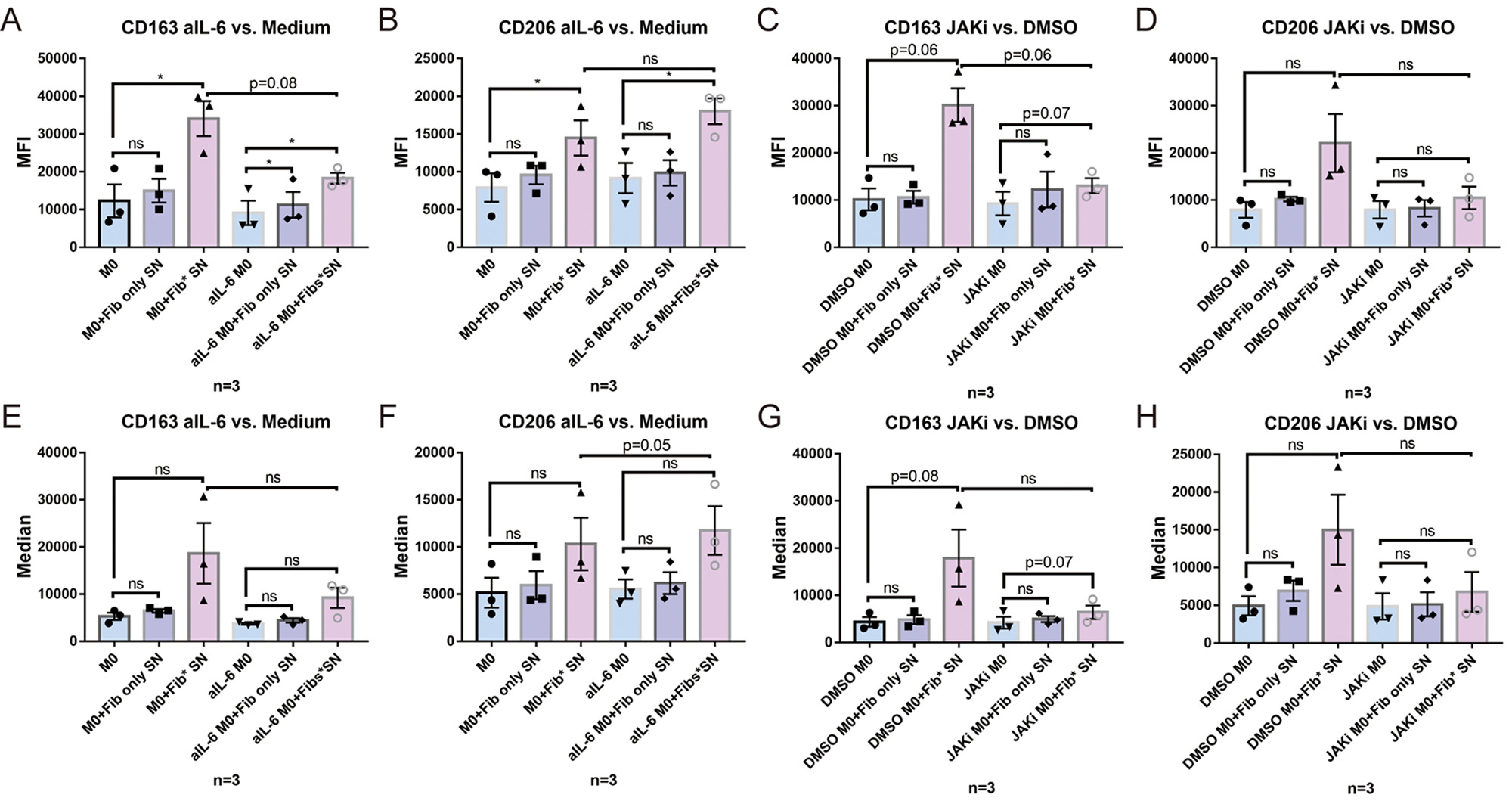
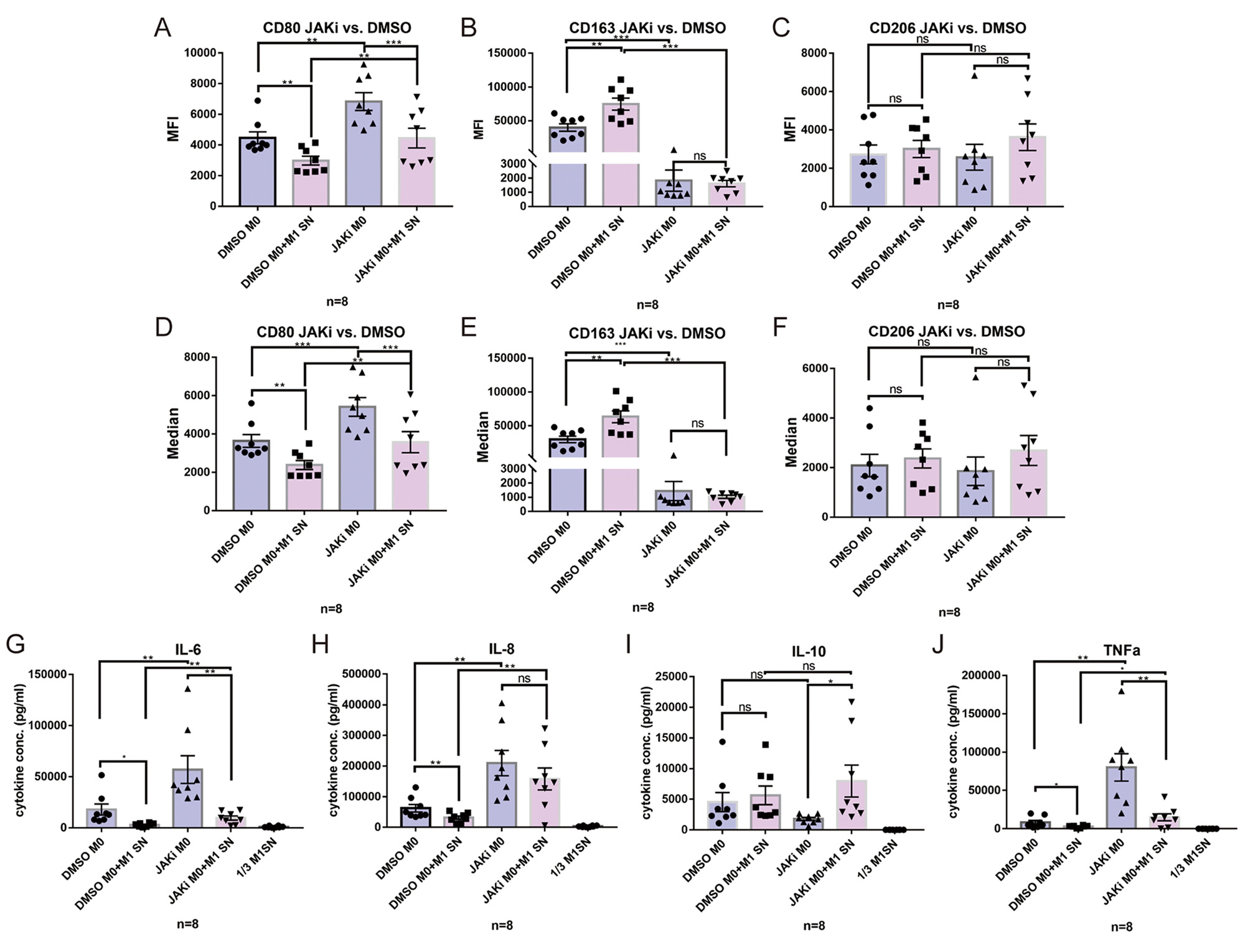
Results: M1-SN had both pro-inflammatory and pro-fibrotic effects on Fib, as evidenced by upregulation of COX-2, aSMA and COL-1. In addition, M1-SN induced a significant upregulation of IL-6 and MMP-3 in Fib. These effects were significantly suppressed in presence of the selective JAK inhibitor (JAKi) Upadacitinib. M2-SN slightly downregulated IL-6, MMP-3, COX-2 and aSMA expression. SN from TNFa/IL-1b pre-stimulated Fib induced a polarization of undifferentiated M0 Mph towards the M2 phenotype as evidenced by expression of high levels of CD163, CD206 and IL-10. Addition of an anti-IL-6 receptor Ab (tocilizumab) and JAKi both inhibited development of this anti-inflammatory phenotype. M0 polarization towards M2 was also induced by M1-SN: CD163 was significantly upregulated by M1-SN, while IL-6, IL-8 and TNFa were evidently downregulated by M1-SN. On the other hand, JAKi forcefully suppressed the effects of M1-SN on M0 Mph.
Conclusion: Polarized Mph have s strong modulatory effect on the Fibs. On the other hand, pre-activated pro-inflammatory Fib and polarized M1 themselves have also a significant effect on Mph polarization, supporting M2 differentiation. JAKi abrogate proinflammatory and profibrotic responses in Fib, while JAKi and anti-IL-6 also inhibited differentiation of Mph into M2, thereby pointing to a new functional aspect of this Immunomodulator.
To cite this abstract in AMA style:
Li J, Cai Y, Guan X, Ewald M, Tykocinski L, Lorenz H, Tretter T. Interactions Between Synovial Fibroblasts and Macrophages: Implications for Tissue Remodeling in Chronic Inflammatory Diseases and Macrophage Differentiation in Response to the Immune Microenvironment [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/interactions-between-synovial-fibroblasts-and-macrophages-implications-for-tissue-remodeling-in-chronic-inflammatory-diseases-and-macrophage-differentiation-in-response-to-the-immune-microenvironment/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/interactions-between-synovial-fibroblasts-and-macrophages-implications-for-tissue-remodeling-in-chronic-inflammatory-diseases-and-macrophage-differentiation-in-response-to-the-immune-microenvironment/