Session Information
Date: Sunday, November 7, 2021
Title: Systemic Sclerosis & Related Disorders – Basic Science Poster (0541–0559)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Suction blister fluid provides a unique opportunity to analyse the dermal microenvironment of SSc. We report an integrated analysis of proteomic data from dermal interstitial fluid in early dcSSc patients compared with healthy controls (HC), and genome-wide transcriptomic analysis of concurrently taken skin biopsies. Interpreting differential protein expression in the context of RNA sequencing (RNAseq) provides unique insight into potential mediators and pathways relevant to SSc.
Methods: The BIOPSY cohort recruited 21 early dcSSc patients with mean skin score (MRSS) of 21 (sd 11) and 16 HCs. Forearm skin blister fluid was obtained using the dermal suction blister method and assayed using the Olink platform (www.olink.com) (1192 proteins). Simultaneous 4mm punch biopsies were taken from all subjects, for genome-wide transcriptomic profiling by RNAseq. Integrated analysis of gene and protein expression data, together with SSc clinical and laboratory characteristics was conducted using WGCNA and clusterProfiler in R. This method clusters individual analytes into modules sharing similar expression patterns. Each module has been arbitrarily assigned as a colour. Modules with significant correlation to early dcSSc diagnosis, and with each other, were identified. Significant genes or proteins within a module were identified by a fold change of at least 1.5 compared to HCs.
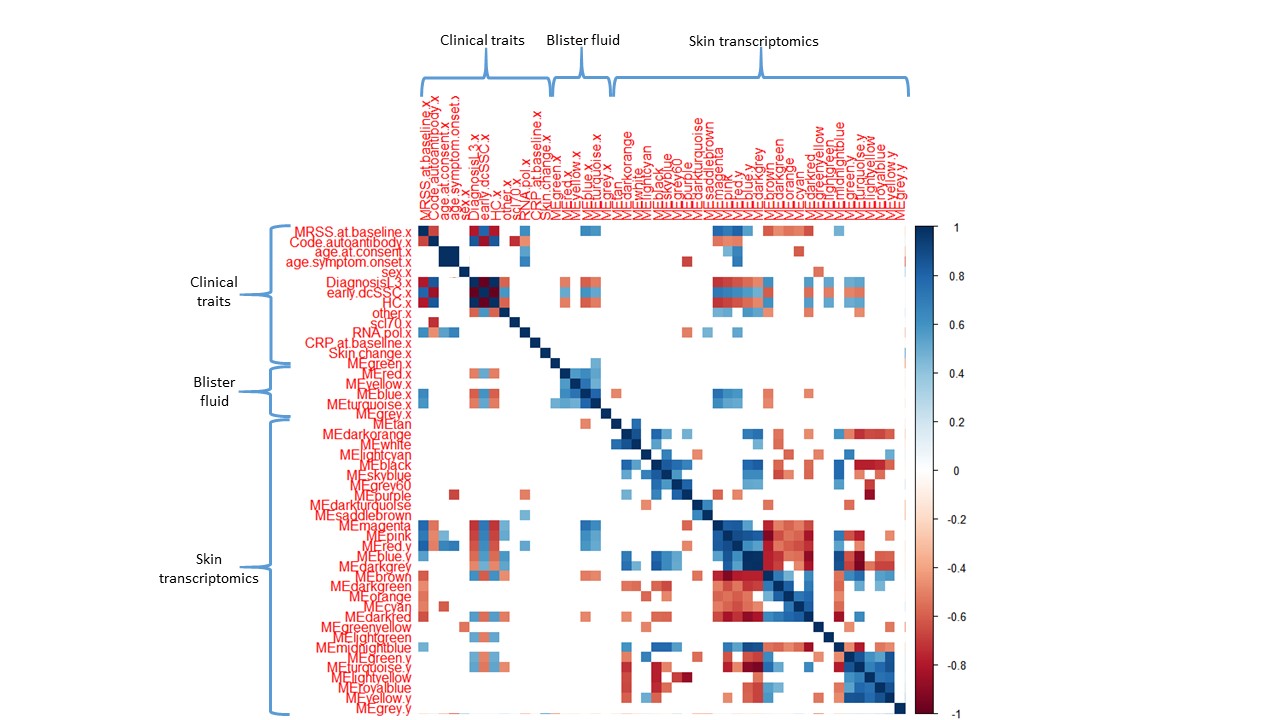
Results: WGCNA identified 6 modules in blister fluid, and 30 modules from skin RNAseq data (Figure 1). The magenta module (385 genes) in the skin correlated most significantly with early dcSSc (r=0.74, p< 0.001), and MRSS at baseline (r=0.82, p< 0.001). The blue module (450 proteins) in the blister fluid correlated most strongly with early dcSSc disease (r=0.6, p< 0.001), MRSS at baseline (r=0.63, p< 0.001), and skin progression (r=0.54, p=0.002). Both modules significantly correlated with each other (r=0.6, p< 0.05).
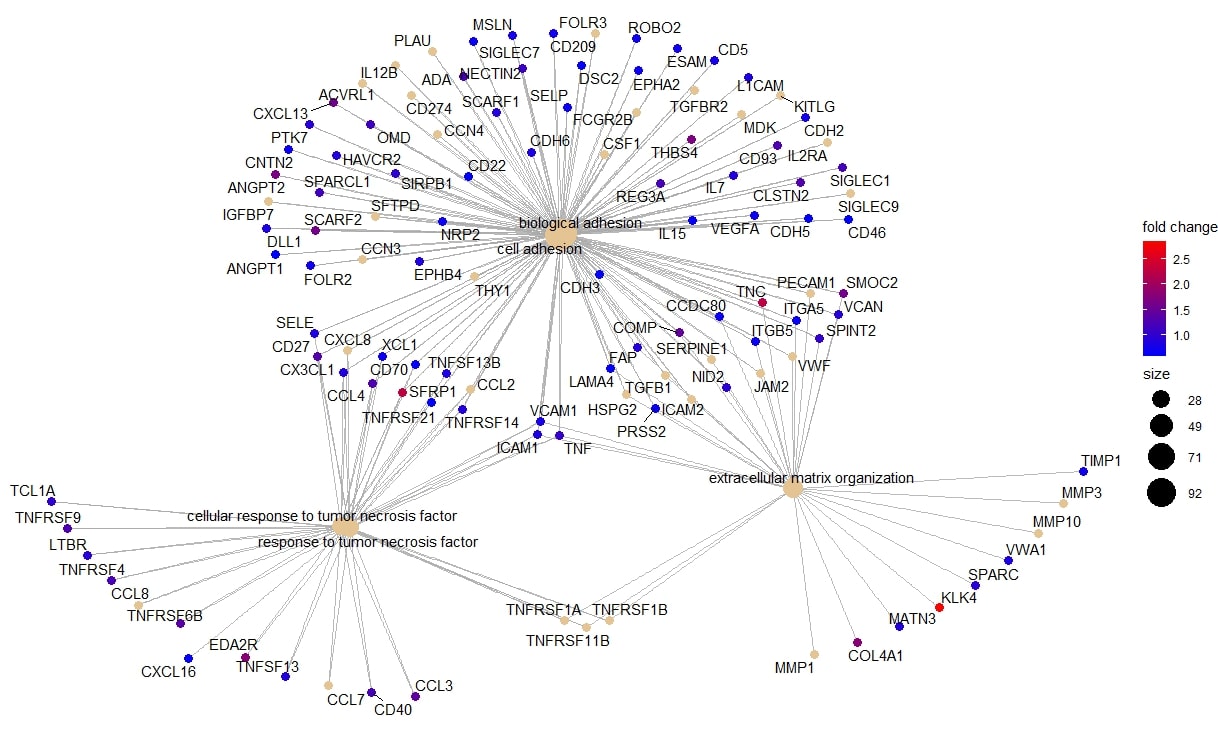
Consistent with current concepts of SSc pathogenesis, key biological processes identified in the magenta module were angiogenesis, extracellular matrix (ECM) structure, and endothelial cell proliferation, with COMP, COL4A4, COL11A1 having the highest fold change compared to HC. The blue module consisted of proteins centred around ECM organisation, cell adhesion, and response to TNF, with upregulation of SFRP1, TNC and KLK4 in early dcSSc compared to HC (Figure 2).
Significant Hallmark pathways were compared between the two modules in each tissue modality and HCs (Figure 3). Notably, some pathways were upregulated in both skin gene expression (magenta) and blister fluid (blue) modules including NFKB signalling, allograft rejection, and KRAS signalling. Significantly activated pathways in magenta (skin gene) but not the blue module (blister fluid protein) included angiogenesis, Hedgehog and TGFβ signalling. Conversely, protein secretion, IFNγ response and mitotic spindle pathways were significantly increased only in the blue module.
Conclusion: the molecular pathology of SSc. Correlation with skin severity or progression suggests potential for developing a novel composite biomarker for skin.
To cite this abstract in AMA style:
Clark K, Csomor E, Campochiaro C, Taylor A, Teo Y, Nevin K, Morse M, Ong V, Derrett-Smith E, Wisniacki N, Flint S, Denton C. Integrated Analysis of Dermal Blister Fluid Proteomics and Genome-wide Skin Gene Expression Gives New Insight into Pathogenesis of Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/integrated-analysis-of-dermal-blister-fluid-proteomics-and-genome-wide-skin-gene-expression-gives-new-insight-into-pathogenesis-of-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/integrated-analysis-of-dermal-blister-fluid-proteomics-and-genome-wide-skin-gene-expression-gives-new-insight-into-pathogenesis-of-systemic-sclerosis/