Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The risk of heart failure (HF) is increased in patients with rheumatoid arthritis (RA), although mechanisms of myocardial dysfunction underlying HF development in RA remain poorly understood. Prior work by others has shown that mice with collagen induced arthritis (CIA) have impaired myocardial function early in the course of disease that is associated with tissue citrullination (Pironti et al. Acta Physiologica 2022). Recent studies by our group have highlighted the role that two post-translational modifications (PTMs), malondialdehyde-acetaldehyde (MAA) and citrulline (CIT), play together in promoting local inflammation and fibrosis in RA synovial and lung tissues. Whether these two PTMs mediate myocardial damage leading to RA-related HF is unknown. This study investigated PTM expression along with extracellular matrix (ECM) proteins in myocardial tissues early in the course of collagen-induced arthritis (CIA).
Methods: Arthritis prone male DBA/1J mice (n=3/group) were subjected to collagen induced arthritis by standard protocols. Identically timed saline injections were used in control mice and all mice were sacrificed at 5 weeks. Hearts were resected and myocardial tissues were paraffin embedded, sectioned, and subjected to immunohistochemistry (IHC) using anti-MAA, anti-CIT, and anti-vimentin (VIM) antibodies. Additional sections were stained with trichrome to quantify total collagen deposition. Mice were assessed for arthritis development using a semi-quantitative score. Statistical comparisons of CIA vs. control mice were performed using Student t-tests, while co-localization of PTMs and ECM proteins was determined using the Fiji plug-in, Coloc 2 in Image J and by generating Pearson’s correlation coefficients (r2).
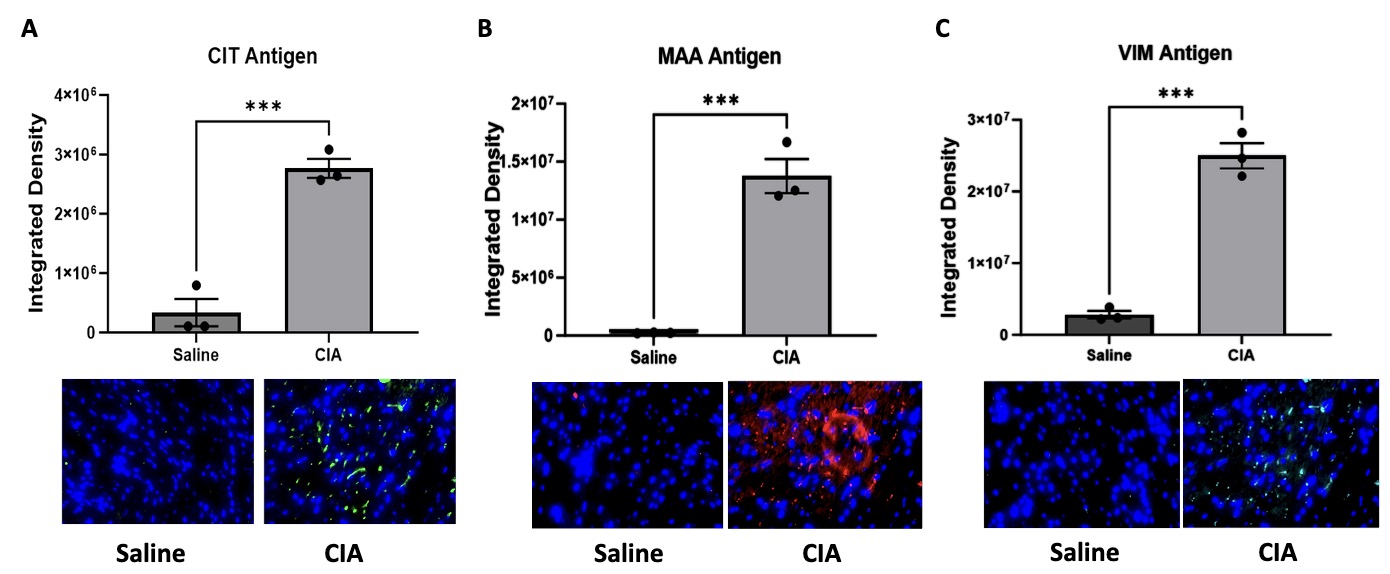
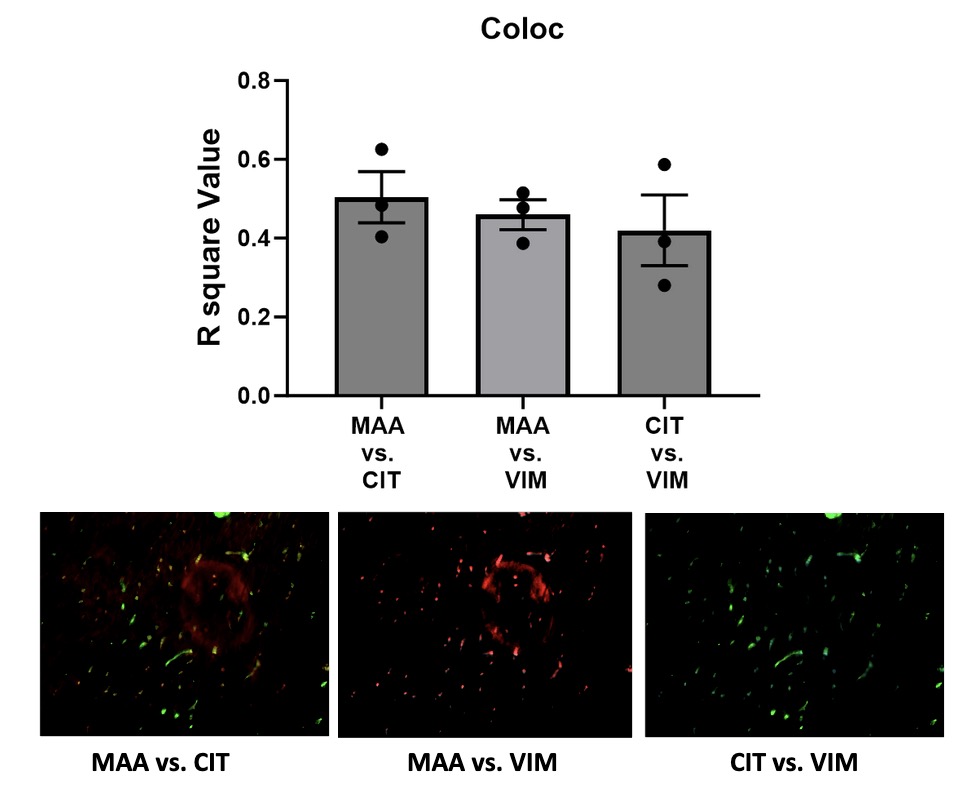
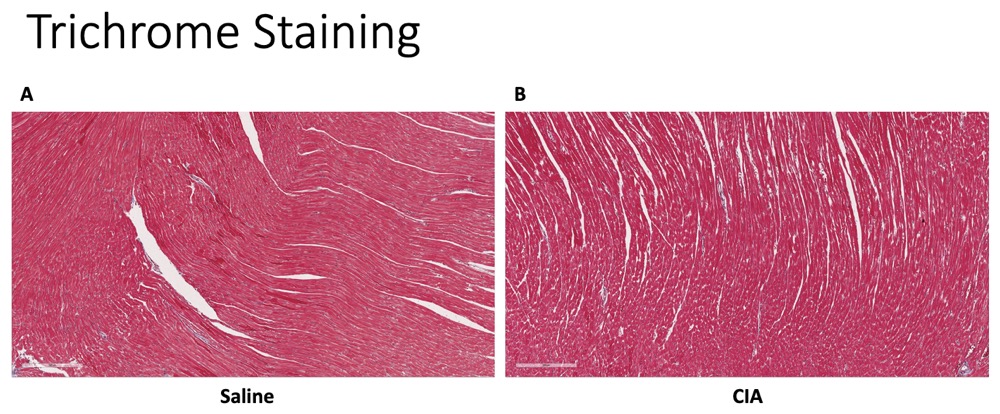
Results: IHC staining revealed significantly increased expression of CIT (p< 0.001), MAA (p< 0.001), and VIM (p< 0.001) antigens in heart tissues of CIA vs. control mice (Fig. 1). There were also moderate levels co-localization demonstrated between MAA-CIT (r2 = 0.50), MAA-VIM (r2 = 0.46), and CIT-VIM (r2 = 0.42) (Fig. 2). In contrast, trichrome staining showed no significant difference in collagen deposition in the hearts of CIA vs. control mice (Fig. 3). Arthritis scores were higher in CIA mice compared to saline injected mice (p< 0.05, data not shown).
Conclusion: This study is the first to demonstrate increased expression and co-localization of MAA-CIT and ECM proteins in myocardial tissues of an early arthritis mouse model. Given prior findings that proteins co-modified with MAA and CIT render both pro-inflammatory and pro-fibrotic effects, these results suggest the presence of these dual modifications in heart tissues, which could play a role in the pathogenesis of myocardial dysfunction in RA. Additional investigations will be needed to quantify the functional consequences of these findings and to examine whether a longer time course of CIA is needed to detect evidence of myocardial fibrosis.
To cite this abstract in AMA style:
Zhou W, Duryee M, Aripova N, Poole J, Hunter C, Nelson A, Johnson T, Anderson D, Thiele G, Mikuls T. Increased Expression of Malondialdehyde-Acetaldehyde and Citrullinated Proteins in Myocardial Tissues During the Early Evolution of Collagen-Induced Arthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/increased-expression-of-malondialdehyde-acetaldehyde-and-citrullinated-proteins-in-myocardial-tissues-during-the-early-evolution-of-collagen-induced-arthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/increased-expression-of-malondialdehyde-acetaldehyde-and-citrullinated-proteins-in-myocardial-tissues-during-the-early-evolution-of-collagen-induced-arthritis/