Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Juvenile-onset systemic sclerosis (jSSc) is a systemic autoimmune disease characterized by vasculopathy and multiorgan fibrosis leading to significant morbidity and early mortality. Autologous stem cell transplant (ASCT) is an emerging mainstay of treatment for severe, medication refractory disease in adults. Data is limited in jSSc. Herein, we report the outcomes of seven jSSc patients 3 months to 4 years after receiving ASCT.
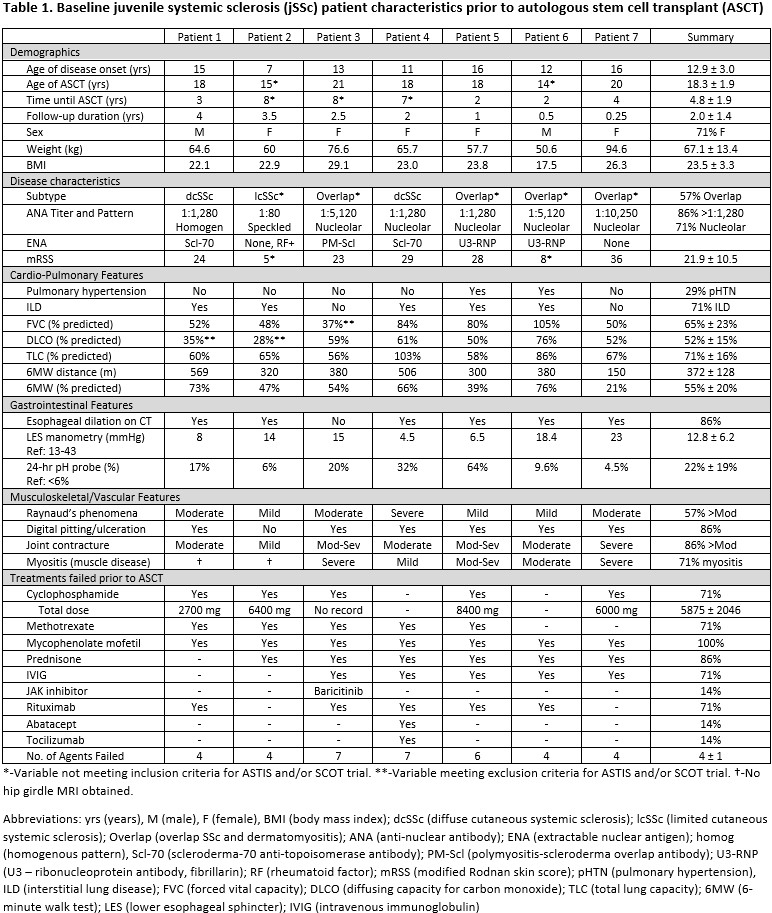
Methods: All patients were referred to our multidisciplinary pediatric scleroderma center, had disease refractory to ≥3 immunosuppressive (IS) therapies, and met ASCT criteria by lung or skin disease severity, or both. These patients would not have met inclusion criteria for more classic adult SSc trials, ASTIS or SCOT, due to longer disease duration, lower DLCO, and disease subtypes. Our ASCT protocol began with stem cell mobilization with cyclophosphamide and filgrastim, collected via pheresis. Conditioning chemoradiation was then administered per an IRB-approved individual treatment plan (Patients 1-3) or per our clinical trial NCT03630211 (Patients 4-7) with rituximab, ATG, total body irradiation 600 cGy with lung and kidney shielding, and cyclophosphamide or thiotepa +/- alemtuzumab. CD34+ selected cryopreserved autologous peripheral blood stem cell graft was infused on day 0. Antimicrobial prophylaxis and viral monitoring (EBV, CMV, adenovirus) followed institutional standards. Corticosteroids were discontinued by week 5 post-ASCT. Clinical outcomes and patient reported measures were collected at baseline (pre-ASCT) and at 6-month intervals post-ASCT.
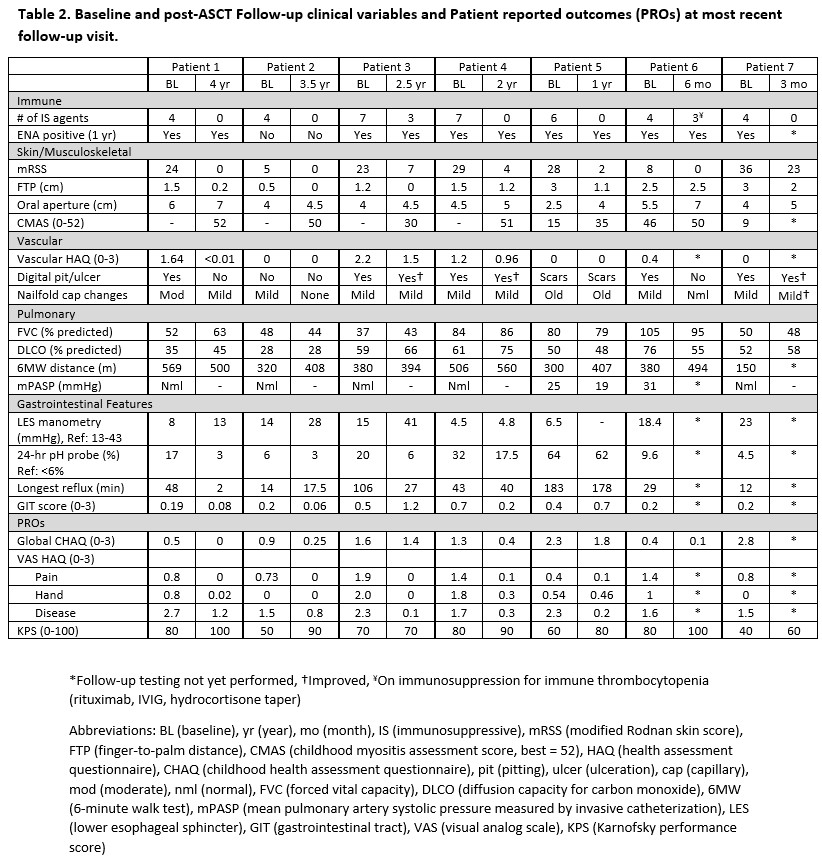
Results: Baseline characteristics are reported in Table 1 and outcomes in Table 2. Mean follow-up time was 2.0 ± 1.4 years (range 0.25-4). All 7 patients engrafted. Median neutrophil and platelet engraftment occurred on days 11 (range 10-27) and 20 (range 16-38) respectively. Global function (CHAQ) improved for all patients by a mean of 0.5 or 58% (range: 12.5-100%) surpassing the MCID 2.5-fold. All patients showed improvement in most or all VAS subdomains. From a BMT standpoint, performance scores increased by a mean of 19 from 66 to 84. Five patients discontinued IS and two reduced the number of agents. All patients met the mRSS MCID, with a mean reduction of 17 or 84% (three with mRSS of 0). Myositis improved in all patients although pre-ASCT childhood myositis score was not available for all. Four patients had repeat lower esophageal manometry with mean improvement of 11 mmHg (range: 0.3-26 mmHg) associated with mean reduction in acid exposure by 50%. FVC and DLCO overall remained stable. 6-minute walk distance improved by a mean of 16% (range: 4-36%). One patient with pulmonary hypertension had normalization of pulmonary pressures. Digital pitting/ulceration improved in 5 patients, stable in 1. There were no significant post-ASCT infectious or cardiac, pulmonary or renal events.
Conclusion: Among our cohort of refractory jSSc patients with moderate to severe disease, ASCT was a safe and effective intervention that provided sustained global disease modifying improvement up to 4 years after transplant with benefit seen as early as 3 months for all patients.
To cite this abstract in AMA style:
Li J, Horvei P, Rosser F, Rose-Felker K, Sood V, Olson A, Vandergrift V, Hogue N, Farver L, Mcguire D, Havrilla H, Alexander J, McIntyre S, Szabolcs P, Torok K. Improvement Across Multi-organ Domains and Patient Reported Outcomes in Refractory Juvenile-Onset Systemic Sclerosis (jSSc) up to 4 Years After Autologous Stem Cell Transplantation (ASCT) [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/improvement-across-multi-organ-domains-and-patient-reported-outcomes-in-refractory-juvenile-onset-systemic-sclerosis-jssc-up-to-4-years-after-autologous-stem-cell-transplantation-asct/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improvement-across-multi-organ-domains-and-patient-reported-outcomes-in-refractory-juvenile-onset-systemic-sclerosis-jssc-up-to-4-years-after-autologous-stem-cell-transplantation-asct/