Session Information
Date: Monday, October 27, 2025
Title: (0978–1006) T Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Regulatory T cells (Tregs) are essential for maintaining immune tolerance and limiting excessive immune responses. However, Tregs show high levels of heterogeneity and plasticity, indicated by the production of IL-17A. In rheumatoid arthritis (RA), IL-17A intensifies synovial inflammation by activating synovial fibroblasts. We aim to characterize Treg heterogeneity in RA patients and examine their functional characteristics and role in synovial inflammation.
Methods: CITE-seq was applied to T cells from four treatment-naïve RA patients and four healthy controls. Unsupervised clustering was performed to identify Treg subpopulations. These subpopulations were verified in PBMCs from 150 RA patients and 30 healthy controls using flow cytometry. A suppression assay was conducted to evaluate the suppressive capacity of the sorted Treg subpopulations. In addition, the cytokine-secreting potential of sorted Treg subpopulations was investigated using ELISA, as well as the effect of interaction with RA synovial fibroblasts on cytokine secretion.
Results: CITE-seq analysis from treatment-naïve early RA patients identified Treg subpopulations based on the presence of HLA-DR and CCR6. We verified the surface expression of HLA-DR and CCR6 on Tregs in PBMCs of patients with established RA, revealing a significant decrease in HLA-DR-CCR6+ Treg cells in RA patients compared to healthy individuals. Significantly fewer HLA-DR-CCR6+ Tregs were detected specifically in RA patients with moderate and severe disease activity. We analysed suppressive function, cytokine secretion, and fibroblast interaction to investigate the functional differences between these subpopulations. We found that all Treg subpopulations suppress proliferation of CD4+ responder cells. In addition, we revealed that activated HLA-DR-CCR6+ Treg exclusively secreted IL-17A. Co-cultures with RA synovial fibroblasts showed that HLA-DR-CCR6+ Tregs induced synovial fibroblast activation, indicated by the secretion of IL-6, IL-8, and MMP-3. Interestingly, IL-17A secretion was increased upon coculturing HLA-DR-CCR6+ Treg and synovial fibroblast , highlighting the potential plasticity of this HLA-DR-CCR6+ Treg subpopulation towards a proinflammatory phenotype.
Conclusion: Regulatory T cell subsets defined by HLA-DR and CCR6 markers were identified across different stages of RA and exhibited notable functional heterogeneity. Despite maintaining suppressive capacity, some subsets, particularly HLA-DR⁻CCR6⁺ Tregs, displayed pro-inflammatory features, including IL-17A secretion and activation of synovial fibroblasts. These findings underscore the complexity of Treg biology in RA and suggest that HLA-DR⁻CCR6⁺ Tregs may paradoxically contribute to disease progression under inflammatory conditions.
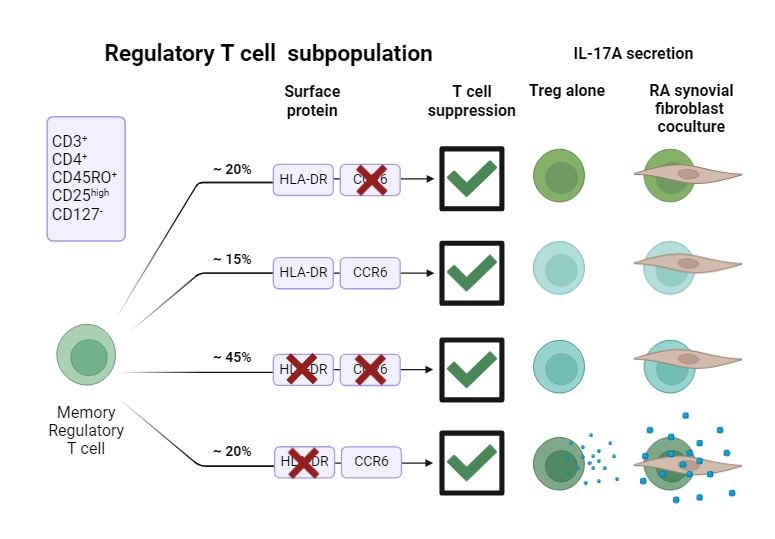 Schematic overview of Treg subpopulation characteristics
Schematic overview of Treg subpopulation characteristics
To cite this abstract in AMA style:
Heeswijk B, Bongenaar M, Davelaar N, de Jong P, den Braanker H, Dolhain R, Lubberts E. IL-17A+ HLA-DR-CCR6+ regulatory T cells: Dual role in T cell suppression and synovial inflammation in arthritis. [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/il-17a-hla-dr-ccr6-regulatory-t-cells-dual-role-in-t-cell-suppression-and-synovial-inflammation-in-arthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/il-17a-hla-dr-ccr6-regulatory-t-cells-dual-role-in-t-cell-suppression-and-synovial-inflammation-in-arthritis/
