Session Information
Date: Monday, November 14, 2022
Title: T Cell Biology and Targets in Autoimmune and Inflammatory Disease Poster
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
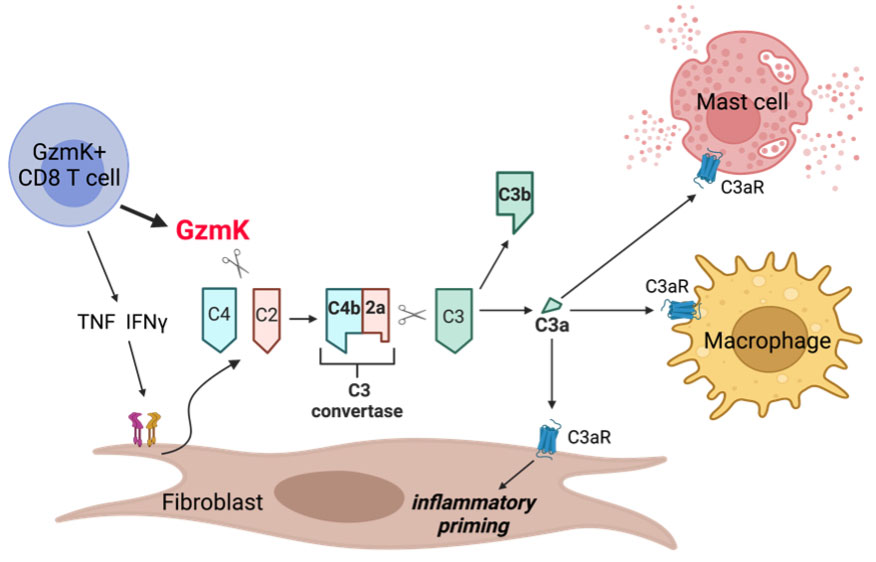
Background/Purpose: T cells are major drivers of rheumatoid arthritis (RA) pathogenesis. Most research has focused on CD4 T cells, but we have found that CD8+ T cells are strikingly abundant in inflamed RA synovium and make more IFNγ and nearly as much TNF as their CD4 T cell counterparts. We have recently identified a new effector CD8+ T cell population abundant in inflamed RA synovium and in other inflamed tissues. Unlike cytotoxic T lymphocytes, these synovial CD8+ T cells have low cytotoxic potential, with low levels of granzyme B and perforin. Instead, these CD8+ T cells expresses high levels of granzyme K (GzmK), a protease that does not cleave caspases. The function of GzmK in inflamed synovium and other tissues is unclear. Here, we describe that GzmK activates a new complement pathway, thereby activating an innate immune cascade with numerous pro-inflammatory effects on surrounding cells.
Methods: We incubated recombinant enzymatically active GzmK with serum-purified complement C2, C3, and/or C4 to investigate the molecular effects of GzmK on complement proteins. We measured cleavage of C2, C3, and C4 using Western blots. We analyzed complement gene expression in a published dataset of bulk RNA-sequencing (RNA-seq) data from fibroblasts, macrophages, B cells, and T cells sorted from human RA and osteoarthritis synovial tissue samples. In addition, we stimulated cultured RA synovial fibroblasts with recombinant IFNγ or TNF for 24 hours, performed low-input RNA-seq, and compared expression levels of selected complement genes. Functional assays include flow cytometry to measure phosphorylation of mammalian target of rapamycin (mTOR) as well as ELISAs to measure cytokine production.
Results: GzmK cleaves C4 to C4b and C2 to C2a to form an active C3 convertase (C4b + C2a) that cleaves C3 to C3a and C3b. Further, we show that synovial fibroblasts, important drivers of chronic inflammation in RA, are the dominant cellular source of complement components in the synovium. IFNγ and TNF, cytokines abundantly produced by CD8 T cells, drive fibroblasts to synthesize and release C2, C3, and C4. Strikingly, we show that GzmK cleaves C4 and C2 secreted by fibroblasts, eliciting formation of an extracellular C3 convertase that generates C3a and C3b. Unlike recent studies of murine synovial fibroblasts, we do not find that human synovial fibroblasts respond to C3a stimulation. Instead, our findings support a three-cell model for complement activation, where CD8 T cell-derived GzmK can act extracellularly on fibroblast-derived complement components, generating C3a and C3b that then acts on other nearby cells, such as macrophages and mast cells (Figure 1).
Conclusion: GzmK activates a novel complement pathway that is independent of the classical, alternative, and lectin pathways of complement activation, and the C3a and C3b that GzmK generates may perpetuate tissue inflammation in RA and other diseases.
To cite this abstract in AMA style:
Jonsson A, Donado C, Gomez-Rivas E, Brenner M. Granzyme K Elicits a New Pathway for Complement Activation in RA Synovium [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/granzyme-k-elicits-a-new-pathway-for-complement-activation-in-ra-synovium/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/granzyme-k-elicits-a-new-pathway-for-complement-activation-in-ra-synovium/