Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Rheumatoid arthritis (RA) is a chronic, systemic inflammatory disease that disproportionately affects females over males (3:1). Although sex-based immune differences have been documented, particularly in neutrophil effector functions, the molecular drivers of sex bias in RA remain unclear. Our lab previously implicated N-formylated peptides, danger-associated molecular patterns from bacteria and mitochondria, in RA pathogenesis via activation of formyl peptide receptor 1 (FPR1) on neutrophils. While the related receptor FPR2 regulates sex differences in steatohepatitis, whether FPR1 mediates sex-specific immune responses in arthritis is unknown.
Methods: Male and female C57/BL6 fpr1+/+ (WT) and fpr1-/- mice were intraperitoneally injected with K/BxN sera at days 0 and 2 for induction of inflammatory arthritis. Joint scores tracking arthritis were recorded by a blinded observer over 7 days. Joints and spleens were harvested for flow cytometry and cytokine expression, respectively. To assess the contribution of hematopoietic versus non-hematopoietic FPR1, bone marrow chimeras were generated by transplanting fpr1-/- marrow into irradiated WT male and female recipients, followed by arthritis induction and scoring over 8 days.
Results: FPR1 knockout and wild-type arthritic mice both showed elevated levels of N-formyl methionine in circulation (Figure 1A). Overall arthritis severity was similar between genotypes (Figure 1B). However, when stratified by sex, male fpr1-/- mice had worse arthritis than wild-type, while female fpr1-/- mice had improved disease (Figure 1C, 1D). There were no significant differences between male and female wild-type mice (Figure 1E). Flow cytometric analysis revealed increased immune cell infiltration in joints of male fpr1-/- mice, whereas no major differences were observed in females (Figure 2A-E) and qPCR showed increased TNFa in male KO mice (Figure 2F). To determine whether FPR1 in hematopoietic cells mediated these effects, we performed bone marrow chimera experiments. Female WT mice receiving fpr1-/- marrow showed no change in arthritis severity compared to controls (Figure 3A). In contrast, male WT mice receiving fpr1-/- marrow developed less severe arthritis than non-chimeric WT males (Figure 3B), suggesting that somatic (non-hematopoietic) FPR1 expression may drive joint inflammation in males.
Conclusion: FPR1 contributes to inflammatory arthritis in a sex- and compartment-specific manner. In females, FPR1 appears to promote inflammation, as its deletion is protective. In males, however, FPR1 deficiency paradoxically worsens arthritis—yet transfer of fpr1-/- bone marrow into WT male hosts reduces disease. These findings suggest that in males, FPR1 expression in non-hematopoietic tissues (e.g., joint stromal or endothelial cells) may exacerbate arthritis, whereas hematopoietic FPR1 may exert a protective effect. Together, our data support a model in which FPR1 plays dual, sex-dependent roles in arthritis pathogenesis, highlighting its potential relevance to understanding sex bias in RA and identifying tissue-specific therapeutic targets.
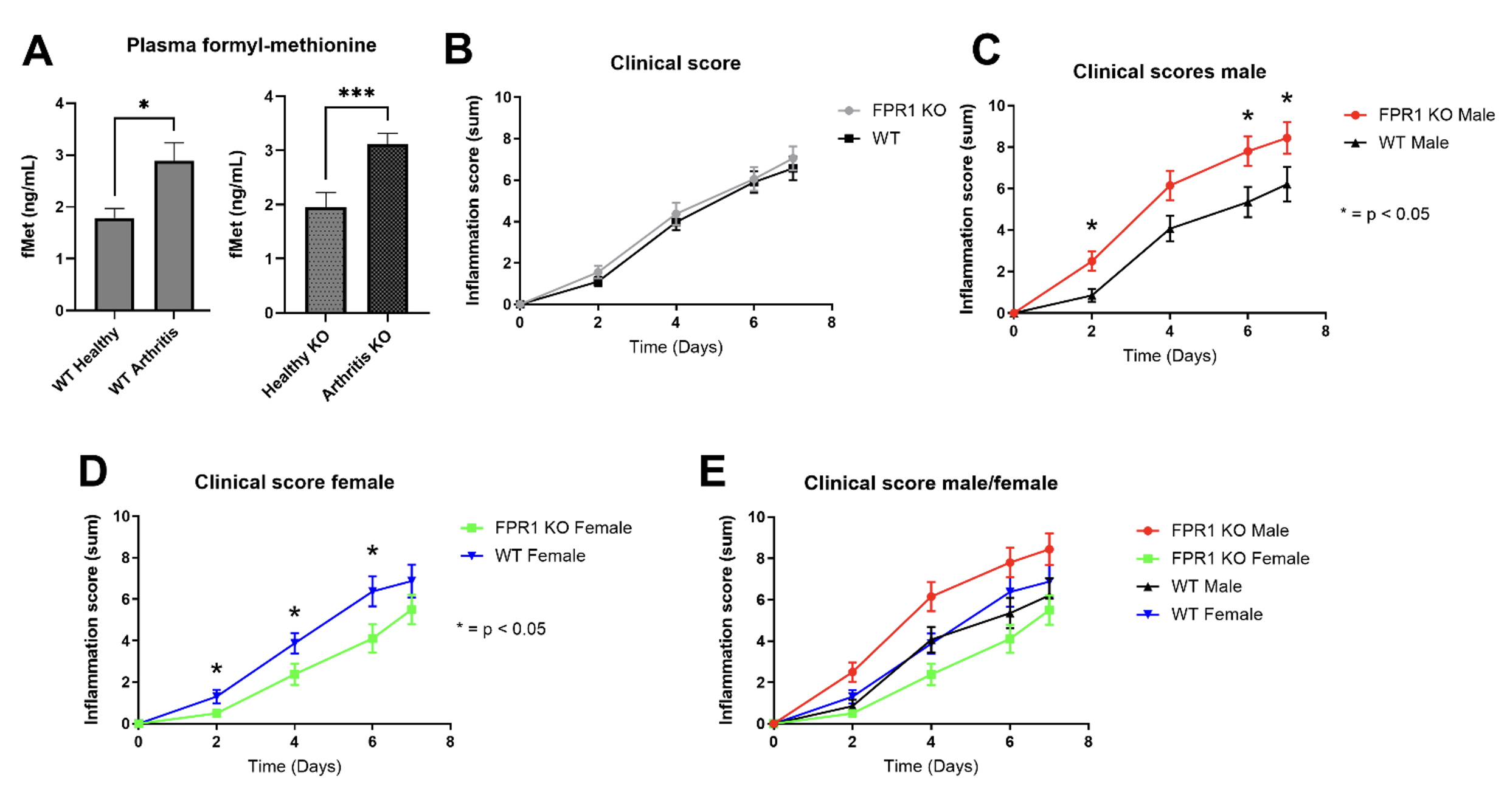 Figure 1. FPR1 worsens arthritis in males while improving arthritis in female mice. Eight to twelve-week-old mice were injected with serum from K/BxN mice on days 0 and 2. Plasma from C57BL/6 wild-type and fpr1-/- (KO) mice at day 0 (WT healthy, Healthy KO) and arthritic wild-type and KO mice at day 7 (WT arthritis, arthritis KO) was analyzed for formylated methionine (fMet) levels by ELISA (A). Mice were followed for 7 days and calculation of paw score (calculated by sum of all 4 paws on scale of arthritis 0-3) was recorded each day by blinded observer. Paw scores were evaluated by segregation by FPR1 genotype (B), genotype and male sex (C), genotype and female sex (D), and all sexes and genotypes (E). n = 10 – 15 mice for each group. * = p < 0.05 based on Mann-Whitney t-test.
Figure 1. FPR1 worsens arthritis in males while improving arthritis in female mice. Eight to twelve-week-old mice were injected with serum from K/BxN mice on days 0 and 2. Plasma from C57BL/6 wild-type and fpr1-/- (KO) mice at day 0 (WT healthy, Healthy KO) and arthritic wild-type and KO mice at day 7 (WT arthritis, arthritis KO) was analyzed for formylated methionine (fMet) levels by ELISA (A). Mice were followed for 7 days and calculation of paw score (calculated by sum of all 4 paws on scale of arthritis 0-3) was recorded each day by blinded observer. Paw scores were evaluated by segregation by FPR1 genotype (B), genotype and male sex (C), genotype and female sex (D), and all sexes and genotypes (E). n = 10 – 15 mice for each group. * = p < 0.05 based on Mann-Whitney t-test.
.jpg) Figure 2. Immune cell infiltration and systemic inflammation are increased in a sex-specific manner mediated by FPR1. On day 7 after induction of serum transfer arthritis in male (M) and female (F) fpr1-/- (KO) and C57BL/6 (WT) mice, mice were sacrificed and one ankle joint was harvested and digested into single cell suspension for flow cytometry. Cells were first selected for CD45 positivity and then stained for markers of T cells (A), activated T cells (B), B cells (C), neutrophils (D), and inflammatory monocytes (E). After sacrifice on day 7, spleens were harvested from all mice for RNA extraction. Quantitative, reverse transcription real-time PCR for TNF-alpha was compared between KO and WT male and female animals (F). * = p < 0.05 based on Mann-Whitney t-test.
Figure 2. Immune cell infiltration and systemic inflammation are increased in a sex-specific manner mediated by FPR1. On day 7 after induction of serum transfer arthritis in male (M) and female (F) fpr1-/- (KO) and C57BL/6 (WT) mice, mice were sacrificed and one ankle joint was harvested and digested into single cell suspension for flow cytometry. Cells were first selected for CD45 positivity and then stained for markers of T cells (A), activated T cells (B), B cells (C), neutrophils (D), and inflammatory monocytes (E). After sacrifice on day 7, spleens were harvested from all mice for RNA extraction. Quantitative, reverse transcription real-time PCR for TNF-alpha was compared between KO and WT male and female animals (F). * = p < 0.05 based on Mann-Whitney t-test.
.jpg) Figure 3. FPR1-deficient bone marrow protects against arthritis in male but not female mice. Arthritis was induced with K/BxN sera in female (A) and male (B) mice as in Figure 1. (A) Female WT mice (blue) were monitored for arthritis severity over 8 days. Bone marrow chimeras were generated by transplant of fpr1-/- bone marrow from female mice into lethally irradiated fpr1+/+ mice. Following engraftment, arthritis was induced with K/BxN serum (KO BM -> WT, green). (B) Similarly to (A), male WT and KO mice were monitored for arthritis severity over 8 days (black and red, respectively) and compared to arthritis development in bone marrow chimera (KO BM -> WT, blue) after successful engraftment and K/BxN sera injection.
Figure 3. FPR1-deficient bone marrow protects against arthritis in male but not female mice. Arthritis was induced with K/BxN sera in female (A) and male (B) mice as in Figure 1. (A) Female WT mice (blue) were monitored for arthritis severity over 8 days. Bone marrow chimeras were generated by transplant of fpr1-/- bone marrow from female mice into lethally irradiated fpr1+/+ mice. Following engraftment, arthritis was induced with K/BxN serum (KO BM -> WT, green). (B) Similarly to (A), male WT and KO mice were monitored for arthritis severity over 8 days (black and red, respectively) and compared to arthritis development in bone marrow chimera (KO BM -> WT, blue) after successful engraftment and K/BxN sera injection.
To cite this abstract in AMA style:
Stultz R, Hermanson P, Mullin N, Ramsey S, Lood C. Formyl Peptide Receptor 1 (FPR1) Influences Arthritis Severity in a Sex- and Compartment-Specific Manner [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/formyl-peptide-receptor-1-fpr1-influences-arthritis-severity-in-a-sex-and-compartment-specific-manner/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/formyl-peptide-receptor-1-fpr1-influences-arthritis-severity-in-a-sex-and-compartment-specific-manner/
