Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Citrulline (CIT) and malondialdehyde-acetaldehyde (MAA) co-adduct native proteins in RA tissues to create a dual pro-inflammatory and pro-fibrotic milieu. Our previous work demonstrated that supernatants from macrophages stimulated with CIT-MAA co-modified fibrinogen induce inflammatory, fibrotic, and invasive phenotypes in synovial and lung fibroblasts. However, the intracellular signaling pathways preceding cytokine release remain poorly defined. The aim of this study was to elucidate the inflammatory signaling pathways in macrophages following stimulation with CIT-MAA co-modified fibrinogen.
Methods: The human monocytic cell line (U-937) was differentiated into macrophages (M0 cells) using phorbol 12-myristate-13-acetate (PMA) and then stimulated with fibrinogen (FIB) that was unmodified, MAA-modified (FIB-MAA), CIT-modified (FIB-CIT), or CIT-MAA co-modified (FIB-CIT-MAA). After 1-hr of antigen stimulation, cells were lysed and protein was analyzed using Western blot analysis targeting the phosphorylated forms and total levels of p38 MAPK, NF-κB (p65), ERK1/2, STAT1, STAT3, STAT6, Akt1, Akt2, JNK, and β-actin. After 48-hrs of stimulation, supernatants were collected and analyzed by ELISA for concentrations of TNFα, IL-1β, IL-6, and MCP-1. After initial testing by Western blot, for each treatment group, there was a replicate group pre-treated with a p38 inhibitor (BIRB-796) or an NF-κB inhibitor (BAY 11-7085) for 1-hr before antigen stimulation. Statistical analysis was performed using one-way ANOVA with Tukey’s multiple comparisons test.
Results: Western blot demonstrated that M0 cells treated with FIB-CIT had significantly increased p38 and NF-κB phosphorylation compared to unmodified FIB, with FIB-CIT-MAA further enhancing phosphorylation of these specific signaling molecules (Fig 1). No significant changes were observed in ERK1/2 and STAT1 following stimulation with any of the antigens. Additionally, phosphorylated Akt1, Akt2, JNK, STAT3, and STAT6 were not detected. Inhibition of signaling pathways after pre-treatment with p38 MAPK and NF-κB inhibitors was validated by Western blot. ELISA revealed that FIB-CIT-MAA stimulation significantly increased secretion of TNF-α, IL-1β, IL-6, and MCP-1 compared to both unmodified FIB, FIB-MAA, and FIB-CIT. Addition of inhibitors to p38 and/or NF-κB decreased release of all 4 cytokines, but only NF-κB inhibition fully abrogated their release (Fig 2).
Conclusion: CIT-MAA co-modified fibrinogen stimulates macrophage activation via the p38 and NF-κB pathways, promoting secretion of pro-inflammatory mediators including TNF-α, IL-1β, IL-6, and MCP-1. Inhibition of these pathways attenuates inflammatory cytokine production, supporting a mechanistic link between CIT-MAA adducts and disease progression in RA. Future research will validate this finding using human monocytes and identify whether agents targeting these post-translational modifications as well as downstream signaling pathways have the potential to attenuate RA progression.
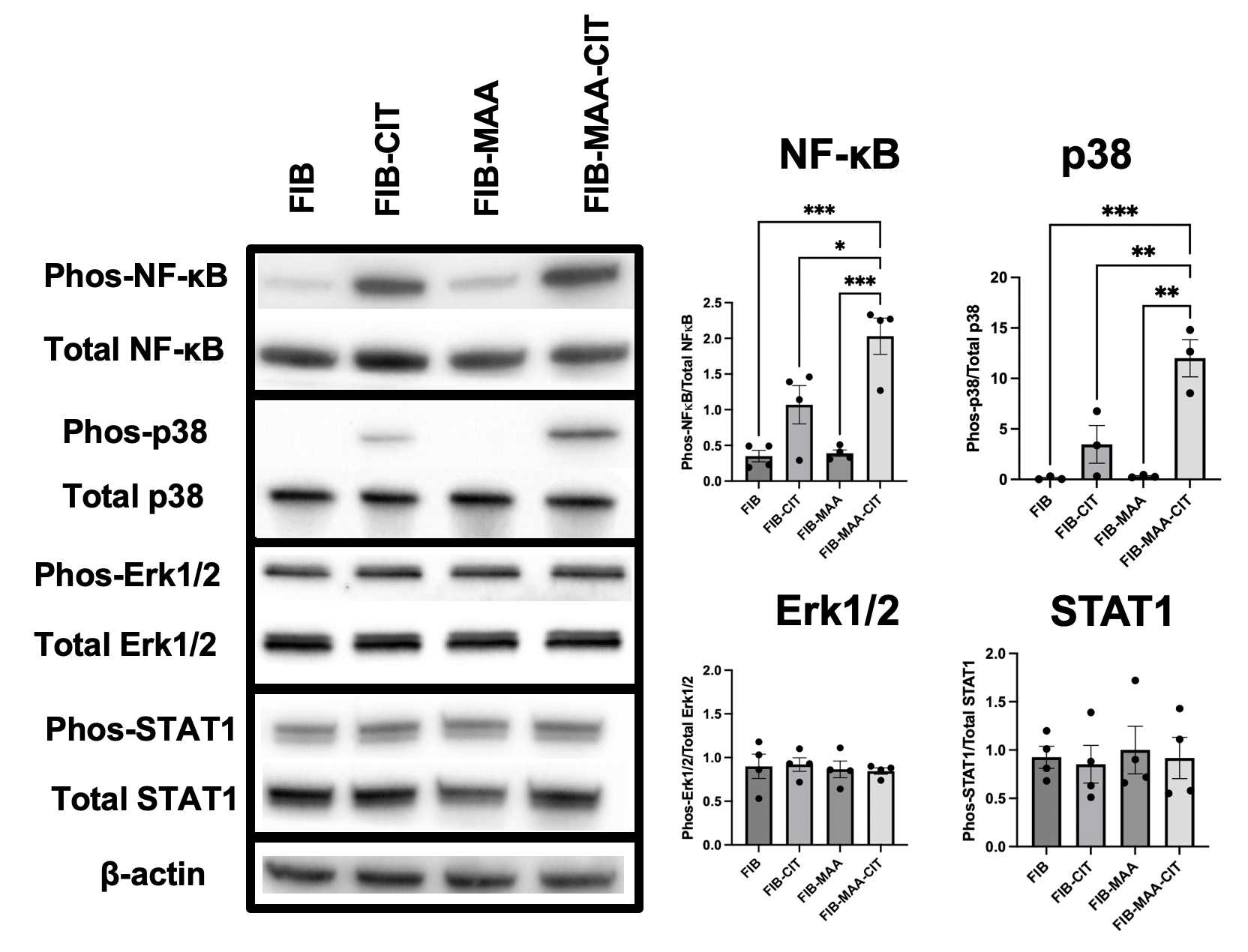 Figure 1. Intracellular Signaling Pathways Activated after Macrophage Stimulation Demonstrated by Western Blot. Macrophages were stimulated for 1-hr with fibrinogen (FIB) that was unmodified or modified with citrulline (CIT) and/or malondialdehyde-acetaldehyde (MAA) prior to measurement of intracellular signaling. Band intensity was quantified using KwikQuant Image Analyzer software. Graphs are plotted as a phosphorylated protein normalized to total protein. Note: phosphorylated Akt1, Akt2, JNK, STAT3, and STAT6 not detected (not shown); One-way ANOVA was performed and the statistical differences between groups are shown in *: * p < 0.05; ** p < 0.01; *** p < 0.001.
Figure 1. Intracellular Signaling Pathways Activated after Macrophage Stimulation Demonstrated by Western Blot. Macrophages were stimulated for 1-hr with fibrinogen (FIB) that was unmodified or modified with citrulline (CIT) and/or malondialdehyde-acetaldehyde (MAA) prior to measurement of intracellular signaling. Band intensity was quantified using KwikQuant Image Analyzer software. Graphs are plotted as a phosphorylated protein normalized to total protein. Note: phosphorylated Akt1, Akt2, JNK, STAT3, and STAT6 not detected (not shown); One-way ANOVA was performed and the statistical differences between groups are shown in *: * p < 0.05; ** p < 0.01; *** p < 0.001.
.jpg) Figure 2. ELISA Demonstrating Decreased Cytokine Production in Macrophages After Inhibition of p38 or NF-κB. 48-hr cytokine concentrations in supernatants were measured for; A) TNFα, B) IL-6, C) IL-1β, and D) MCP-1. One-way ANOVA was performed and the statistical differences between groups are shown in *: * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001.
Figure 2. ELISA Demonstrating Decreased Cytokine Production in Macrophages After Inhibition of p38 or NF-κB. 48-hr cytokine concentrations in supernatants were measured for; A) TNFα, B) IL-6, C) IL-1β, and D) MCP-1. One-way ANOVA was performed and the statistical differences between groups are shown in *: * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001.
To cite this abstract in AMA style:
Johnson H, Zhou W, Duryee M, Hunter C, Thiele G, Mikuls T. Fibrinogen Co-Modified with Malondialdehyde-Acetaldehyde and Citrulline Promotes Pro-Inflammatory Macrophage Differentiation Through p38 and NF-κB Signaling [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/fibrinogen-co-modified-with-malondialdehyde-acetaldehyde-and-citrulline-promotes-pro-inflammatory-macrophage-differentiation-through-p38-and-nf-%ce%bab-signaling/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/fibrinogen-co-modified-with-malondialdehyde-acetaldehyde-and-citrulline-promotes-pro-inflammatory-macrophage-differentiation-through-p38-and-nf-%ce%bab-signaling/
