Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Rheumatoid arthritis (RA) is a systemic autoimmune disease with synovial inflammation as the main pathological feature, and can eventually lead to irreversible joint or organ damage. Recently, several studies have reported that ferroptosis was involved in the pathogenesis of RA. However, the role of ferroptosis in abnormal activation of RA synovial fibroblasts (RASFs) is poorly understood. The purpose of our study was to explore the effect of fatty acid synthase (FASN) on ferroptosis in RASFs.
Methods: FASN expression was assessed by quantitative polymerase chain reaction (qPCR), western blotting and immunohistochemistry in synovial tissues and SFs from RA patients, osteoarthritis (OA) patients and collagen induced arthritis (CIA) mice. SF activation was evaluated by qPCR, CCK-8 and wound healing assay. Lipid peroxidation was detected by MDA levels and C11-BODIPY 581/591 fluorescence intensity. and transient small interfering RNA knockdown were performed to examine expression of SLC7A11 and signaling pathway protein.
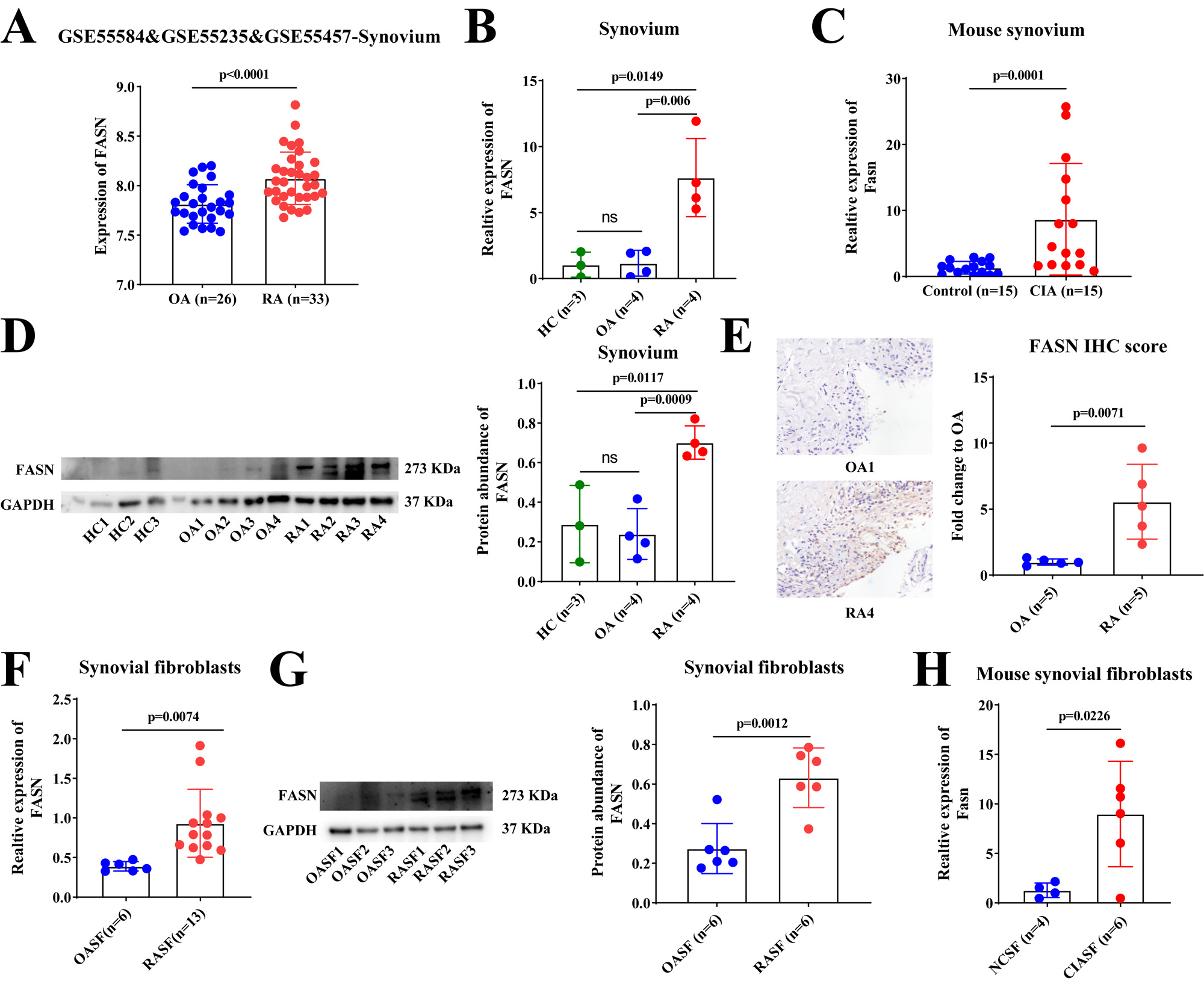
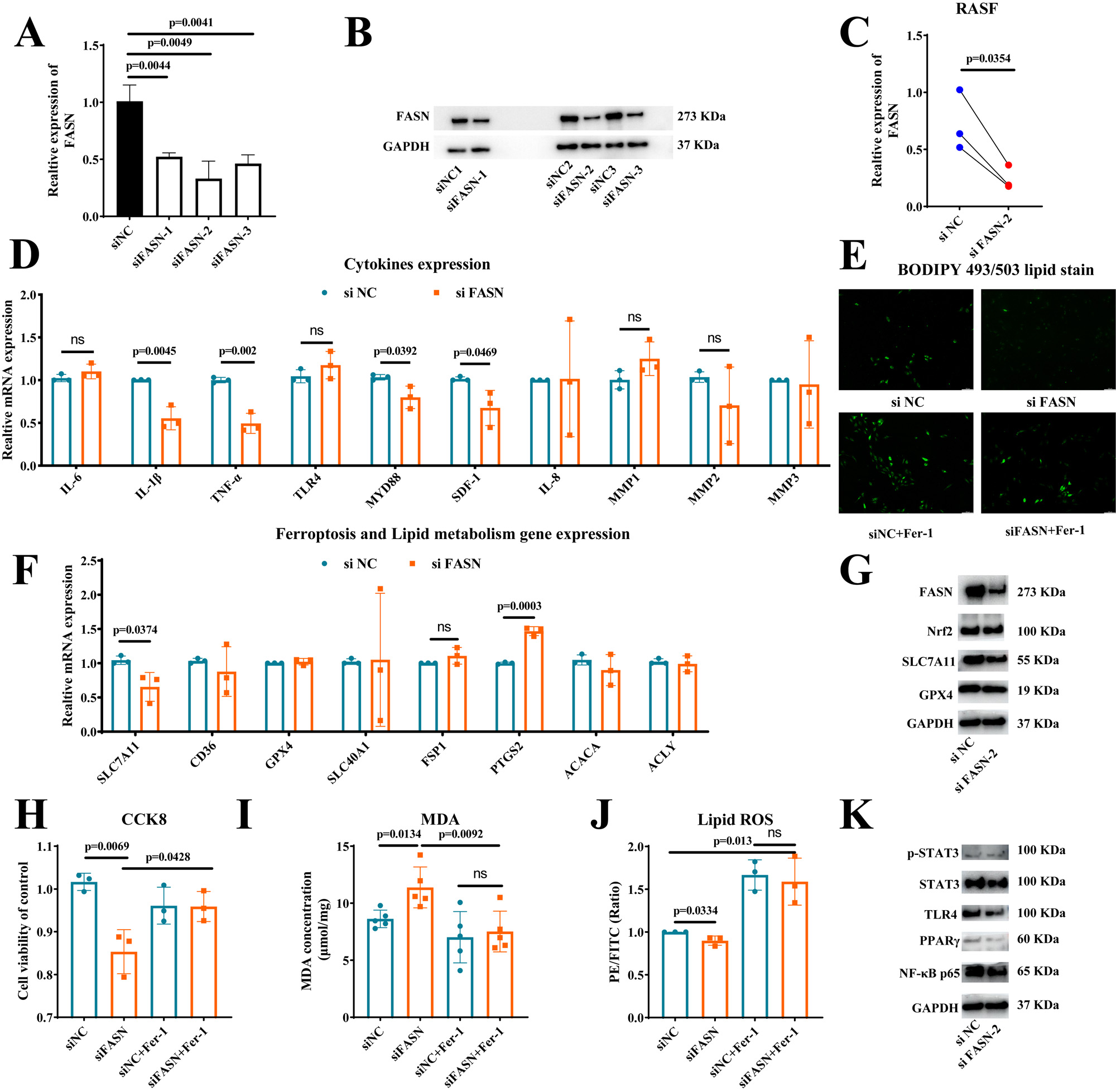
Results: FASN was found to be significantly higher expressed in synovial tissue and SF from RA patients and CIA mice (Figure 1). Silencing of FASN by siRNA in RASFs reduced expression of FASN, SLC7A11, inflammatory cytokines (IL-1β, TNF-α and SDF-1), level of lipid and cell viability, and enhanced the expression of PTGS2 and level of lipid peroxidation, but not affected the expression of other ferroptosis (SLC40A1, FSP1 and GPX4) and lipid (ACACA, ACLY and CD36) genes (Figure 2A-2J). Moreover, treatment with the ferroptosis inhibitor, Ferrostatin-1 (Fer-1) could reverse the effect of FASN knockdown on lipid synthesis, cell viability and ferroptosis in RASFs (Figure 2E-2J). Mechanistically, FASN may promote inflammatory cytokines expression by TLR4/MYD88/NF-κ B signaling pathway, and enhance SLC7A11 expression by phosphorylating the STAT3 signal to suppress ferroptosis of RASFs (Figure 2K).
Conclusion: Our study found that FASN was a ferroptosis suppressor in RASFs. Silencing of FASN inhibited the abnormal activation of RASFs by suppressing STAT3/SLC7A11 axis and the TLR4/MYD88/NF-κB signaling pathway. Target FASN and ferroptosis may serve as a promising novel therapeutic strategy for RA.
To cite this abstract in AMA style:
Cheng Q, chen M, chen X, Xie Y, Du Y, wu H. Fatty Acid Synthase Is a Critical Repressor of Ferroptosis in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/fatty-acid-synthase-is-a-critical-repressor-of-ferroptosis-in-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/fatty-acid-synthase-is-a-critical-repressor-of-ferroptosis-in-rheumatoid-arthritis/