Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Patients with rheumatoid arthritis (RA) experience increased morbidity and mortality due to heart failure with preserved ejection fraction (HFpEF), a condition characterized by diastolic dysfunction largely driven by cardiac fibrosis. However, the underlying mechanisms of HFpEF in RA remain poorly understood. Recent studies from our group have identified the synergistic role of post-translational modifications (PTMs)—specifically citrulline (CIT) and malondialdehyde-acetaldehyde (MAA)—in promoting local inflammation and fibrosis in the joints and lungs of RA patients. Furthermore, these PTMs are increasingly expressed and co-localized in heart tissues of mice with early collagen-induced arthritis (CIA), indicating their potential role in promoting cardiac fibrosis and dysfunction. Therefore, the purpose of this study was to evaluate the temporal relationship between the cardiac expression of these PTMs and cardiac fibrosis in CIA mice.
Methods: Arthritis prone male DBA/1J mice were subjected to CIA (n=8) by standard protocols with saline injections used as control (n=6). Three mice per group were sacrificed at 5 weeks (early CIA) with the remainder sacrificed at 10 weeks (chronic CIA). Prior to being euthanized at 10 weeks, transthoracic echocardiography was performed to assess diastolic function (early to late transmitral diastolic flow [E:A] and myocardial relaxation [E’:A’] velocity ratios) and systolic function (left ventricular ejection fraction %). Additionally, bovine serum albumin coupled to fluorescein isothiocyanate (BSA-FITC) was injected into the left ventricle immediately before sacrifice to visualize microvascular leakage. Hearts were resected and myocardial tissues were paraffin-embedded, sectioned, and stained with trichrome to quantify collagen deposition. Statistical comparisons of functional and morphologic features in CIA vs. control mice were performed using Student’s t-test.
Results: Though CIT and MAA expression were increased and co-localized in heart tissues from mice with early CIA, (Zhou et al. ACR Convergence 2024, Abst 0032), there were no differences in myocardial collagen deposition in early CIA vs. control mice (Fig. 1A). In contrast, we observed significantly increased perivascular and interstitial collagen deposition in chronic CIA vs. control mice (Fig. 1B). Mice with chronic CIA also had increased microvascular leakage (Fig. 2), impaired diastolic function (Fig. 3), and diminished systolic function (Fig. 3) vs. controls.
Conclusion: This study is the first to demonstrate that an increase in CIT and MAA deposition in heart tissues precedes the onset of cardiac fibrosis, microvascular leakage and myocardial dysfunction in mice with CIA. Coupled with our recent studies revealing that CIT and MAA synergistically promote the activation of fibroblasts and endothelial cells in vitro, our findings suggest that CIT and MAA together may be significant drivers of cardiac dysfunction in RA. Further research will explore whether inhibitors of CIT, MAA, or their downstream signaling pathways can attenuate cardiac dysfunction in RA.
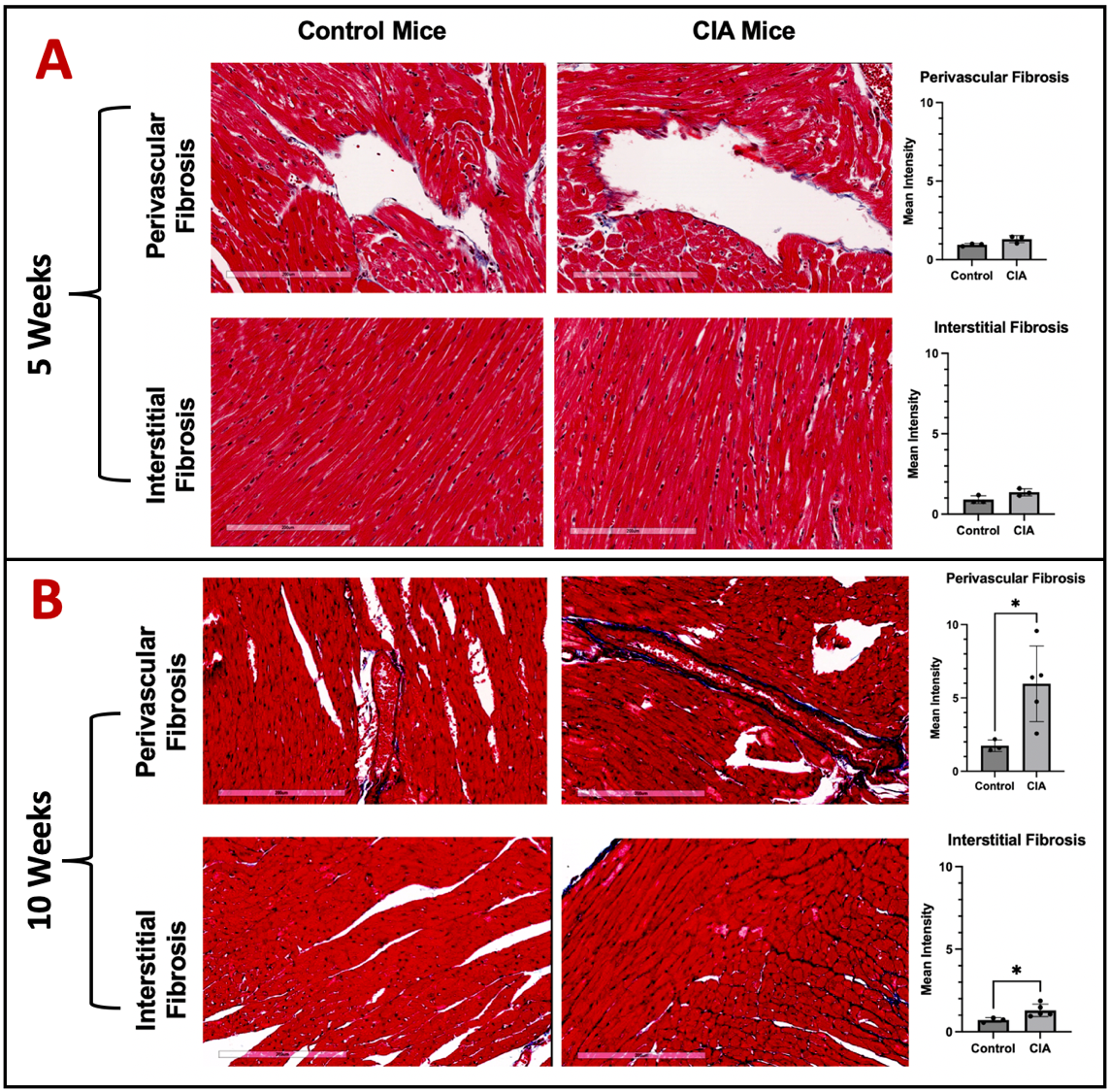 Figure 1. Trichrome Stain Demonstrated Increased Cardiac Fibrosis in CIA Mice at Week 10. Representative image of 20X frame at left ventricle was shown. White scale bar at the bottom indicates 200 μm. Tissue fibrosis (blue) was quantified for control and CIA mice A) 5 weeks and B) 10 weeks post injection. Data shown on graphs are mean ± standard deviation.
Figure 1. Trichrome Stain Demonstrated Increased Cardiac Fibrosis in CIA Mice at Week 10. Representative image of 20X frame at left ventricle was shown. White scale bar at the bottom indicates 200 μm. Tissue fibrosis (blue) was quantified for control and CIA mice A) 5 weeks and B) 10 weeks post injection. Data shown on graphs are mean ± standard deviation.
.jpg) Figure 2. BSA-FITC Revealed Increased Microvascular Leakage in CIA Mice at Week 10. n=1 per group. Representative image of 20X frame at left ventricle was shown. Red arrow points to the sites of micro-vessels with FITC leakage. White scale bar at the bottom indicates 200 μm.
Figure 2. BSA-FITC Revealed Increased Microvascular Leakage in CIA Mice at Week 10. n=1 per group. Representative image of 20X frame at left ventricle was shown. Red arrow points to the sites of micro-vessels with FITC leakage. White scale bar at the bottom indicates 200 μm.
.jpg) Figure 3. Echocardiography of Collagen Induced Arthritis (CIA) and Control Mice at Week 10. Echocardiography was performed on CIA (n=2) and saline-injected Control Mice (n=2) 10 weeks after the first injection. Raw Echocardiographic data was obtained using Pulse-wave, tissue Doppler, and M-mode, and representative images were shown for: A) Control mice; B) CIA mice. These images were used to measure C) E:A ratio, D) E’:A’ ratio, and E) Ejection fraction, respectively. Low E:A ratio and E’:A’ ratio (normal range 1.52 ± 0.40) indicates diastolic dysfunction, and low ejection fraction (normal range 71 ± 11) indicates systolic dysfunction. Data shown on graphs are mean ± standard deviation.
Figure 3. Echocardiography of Collagen Induced Arthritis (CIA) and Control Mice at Week 10. Echocardiography was performed on CIA (n=2) and saline-injected Control Mice (n=2) 10 weeks after the first injection. Raw Echocardiographic data was obtained using Pulse-wave, tissue Doppler, and M-mode, and representative images were shown for: A) Control mice; B) CIA mice. These images were used to measure C) E:A ratio, D) E’:A’ ratio, and E) Ejection fraction, respectively. Low E:A ratio and E’:A’ ratio (normal range 1.52 ± 0.40) indicates diastolic dysfunction, and low ejection fraction (normal range 71 ± 11) indicates systolic dysfunction. Data shown on graphs are mean ± standard deviation.
To cite this abstract in AMA style:
Zhou W, Johnson H, Duryee M, Namvaran A, Garcia J, Hunter C, Johnson T, Anderson D, Bidasee K, Thiele G, Mikuls T. Expression and Co-Localization of Malondialdehyde-Acetaldehyde and Citrullinated Proteins in Myocardial Tissues Precedes the Development of Cardiac Fibrosis in Collagen-Induced Arthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/expression-and-co-localization-of-malondialdehyde-acetaldehyde-and-citrullinated-proteins-in-myocardial-tissues-precedes-the-development-of-cardiac-fibrosis-in-collagen-induced-arthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/expression-and-co-localization-of-malondialdehyde-acetaldehyde-and-citrullinated-proteins-in-myocardial-tissues-precedes-the-development-of-cardiac-fibrosis-in-collagen-induced-arthritis/
