Session Information
Date: Sunday, November 12, 2023
Title: (0066–0095) T Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Interleukin 18 (IL-18) is an inflammasome-activated cytokine that canonically amplifies interferon-gamma (IFNg) production and cytotoxicity by CD8 T-cells (CD8Ts) and Th1 CD4 T-cells (CD4Ts). It is inhibited by the circulating high affinity antagonist, IL-18 binding protein (Il18bp). In certain contexts, IL-18 amplifies non-Th1 responses including Th2, Th17, and Treg. Despite its likely pathogenic role in autoinflammation, clinical observations suggest a possible immunoregulatory role in CD4T-mediated autoimmunity. We sought to understand the role of excess IL-18 in the mixed Th1/17 experimental autoimmune encephalomyelitis (EAE) model of central nervous system autoimmunity.
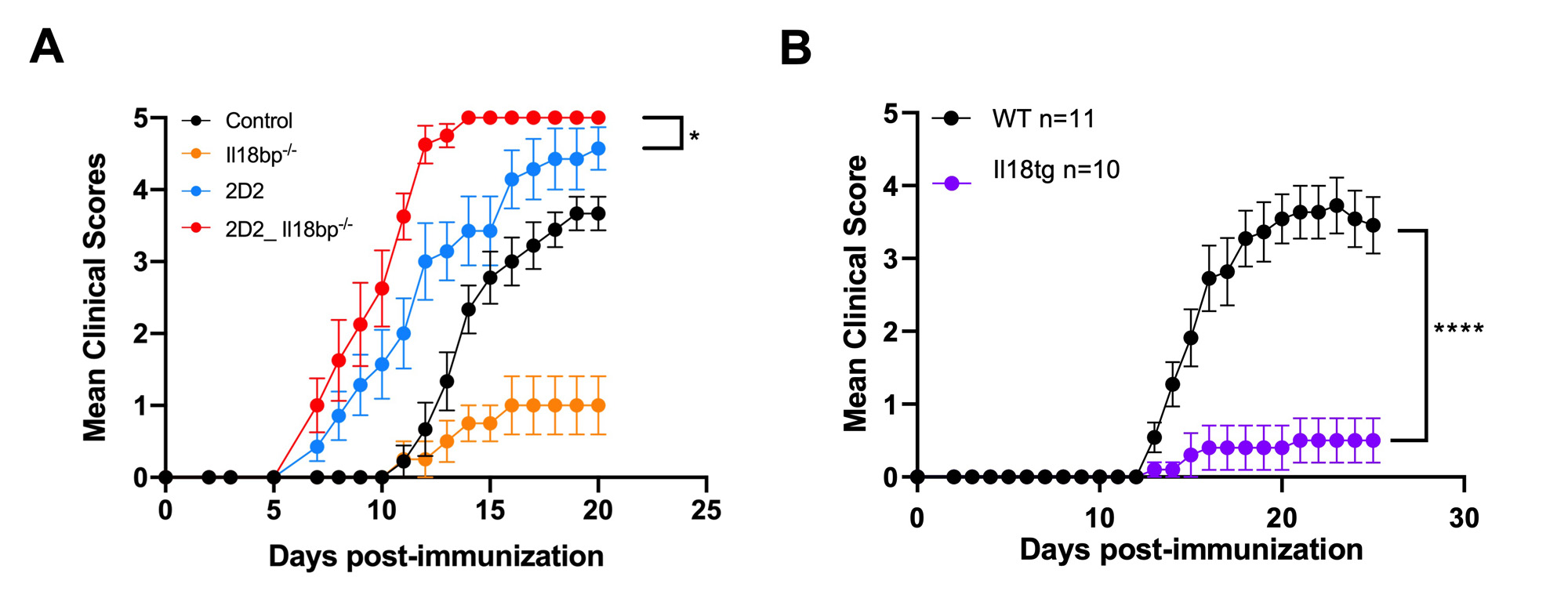
Methods: EAE was induced via immunization with a complete Freund’s adjuvant/Myelin Oligodendrocyte Glycoprotein peptide (MOG35-55) emulsion and pertussis toxin in the following mice: mice deficient in Il-18bp (Il18bp-/-), with transgenic overproduction of mature IL-18 (Il18tg), transgenic for a T-cell receptor recognizing MOG35-55 (2D2 mice), and relevant controls . EAE was monitored by daily clinical scoring of ascending paralysis. Cell number and cellular protein phenotype were determined by flow cytometry.
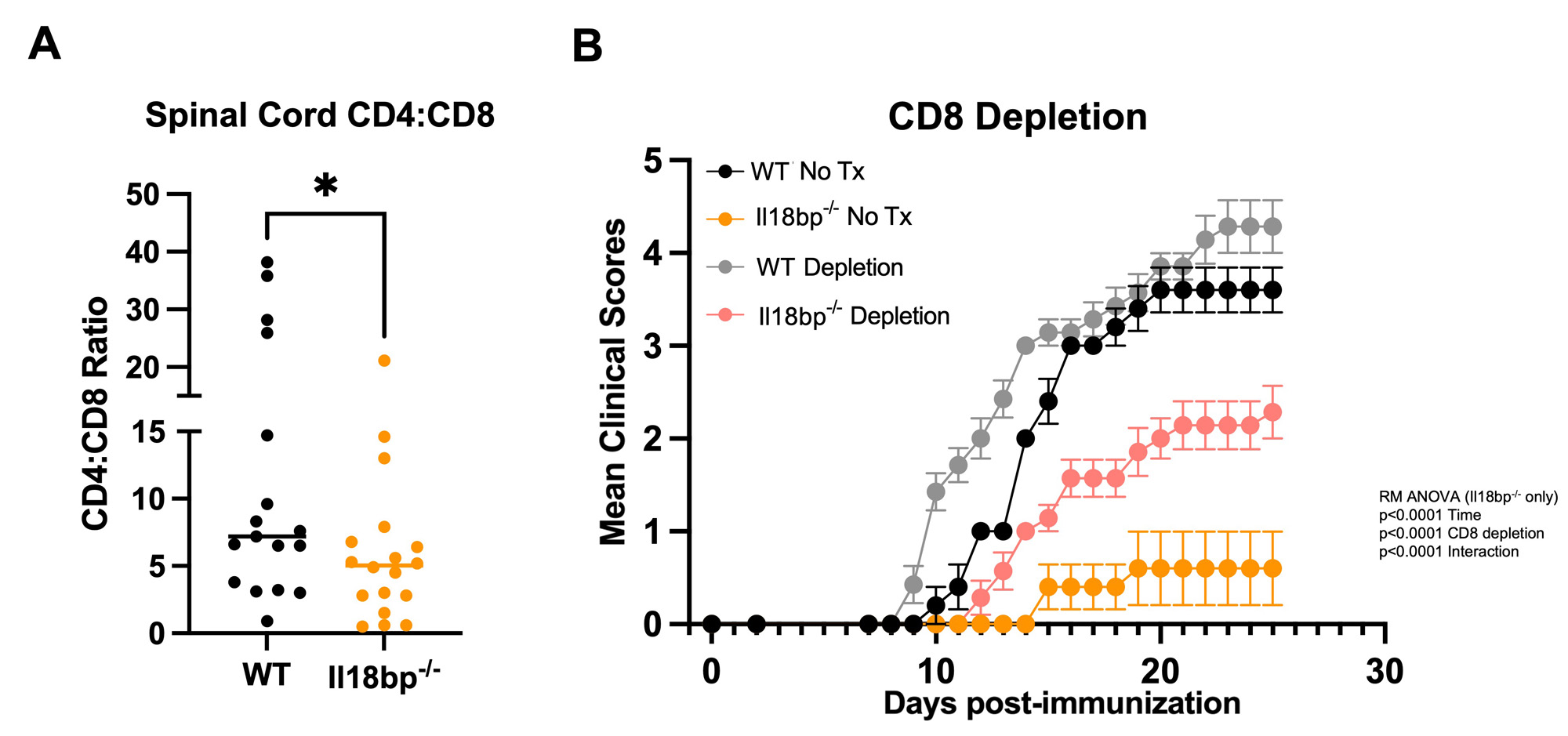
Results: We hypothesized that systemic excess IL-18, would amplify autoreactive T-cell activation and lead to more severe EAE, but both Il18bp-/- and Il18tg mice were profoundly protected from EAE (Fig. 1). Protection from EAE in Il18bp-/- mice depended on IFNg. Draining lymph nodes and spleens from Il18bp-/- mice showed a modest increase in IFNg-producing CD4Ts and equivalent IL-17-producing CD4Ts. Spinal cords demonstrated fewer CD4T cells but comparable markers of Th function/polarization on a per-cell basis. There was increased infiltration of CD8Ts relative to CD4Ts in spinal cords of Il18bp-/-mice (Fig. 2A). 2D2 mice have an abundance of MOG autoreactive CD4Ts and very few CD8Ts (due to allelic exclusion). Paradoxically, Il18bp-/-;2D2 mice developed more severe EAE than control 2D2 mice (Fig.1A), suggesting that an increase in precursor autoreactive CD4Ts and/or loss of CD8Ts switches the effect of excess IL-18 from protective to pathogenic. Sensitivity to EAE was restored in Il18bp-/-mice only after transfer of >5 million autoreactive 2D2 CD4Ts. Further, ex vivo culture with IL-18 improved CD8Ts ability to inhibit EAE while CD8-depletion substantially, but incompletely, diminished protection in Il18bp-/- mice (Fig.2B).
Conclusion: These data suggest the IL-18 can amplify both autoreactive CD4T and previously described CD8 T suppressor cells but the latter dominate with a physiologic T cell repertoire. Excess IL-18 may function to preferentially augment CD8T suppressor function to clear autoreactive CD4T and mediate protection in EAE. This may inform novel strategies to amplify endogenous suppressive T cells or enhance cellular therapeutics in the treatment of autoimmune diseases.
To cite this abstract in AMA style:
Morrissette J, Dang V, Varghese J, Lanzar Z, Nguyen J, Landy E, Frank-Kamenetskii A, Canna S. Excess IL-18 Protects from Experimental Autoimmune Encephalomyelitis Mainly via Preferential Augmentation of Suppressor/Regulatory over Autoreactive T-cell Function [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/excess-il-18-protects-from-experimental-autoimmune-encephalomyelitis-mainly-via-preferential-augmentation-of-suppressor-regulatory-over-autoreactive-t-cell-function/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/excess-il-18-protects-from-experimental-autoimmune-encephalomyelitis-mainly-via-preferential-augmentation-of-suppressor-regulatory-over-autoreactive-t-cell-function/