Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Ankylosing spondylitis (AS) is a chronic inflammatory disease primarily affecting the axial skeleton. T helper 17 (Th17) cells play a pivotal role in the pathogenesis of AS, characterized by their production of IL-17. However, not all Th17 cells contribute equally to disease progression. Pathogenic Th17 (pTh17) cells, distinguished by their ability to simultaneously produce multiple pro-inflammatory cytokines, such as IL-17A, IFN-γ, and GM-CSF, are particularly implicated in severe inflammation. These polyfunctional pTh17 cells drive chronic inflammation and are associated with enhanced disease activity in AS. Identifying specific molecular markers and therapeutic targets for these cells could offer new avenues for treatment.
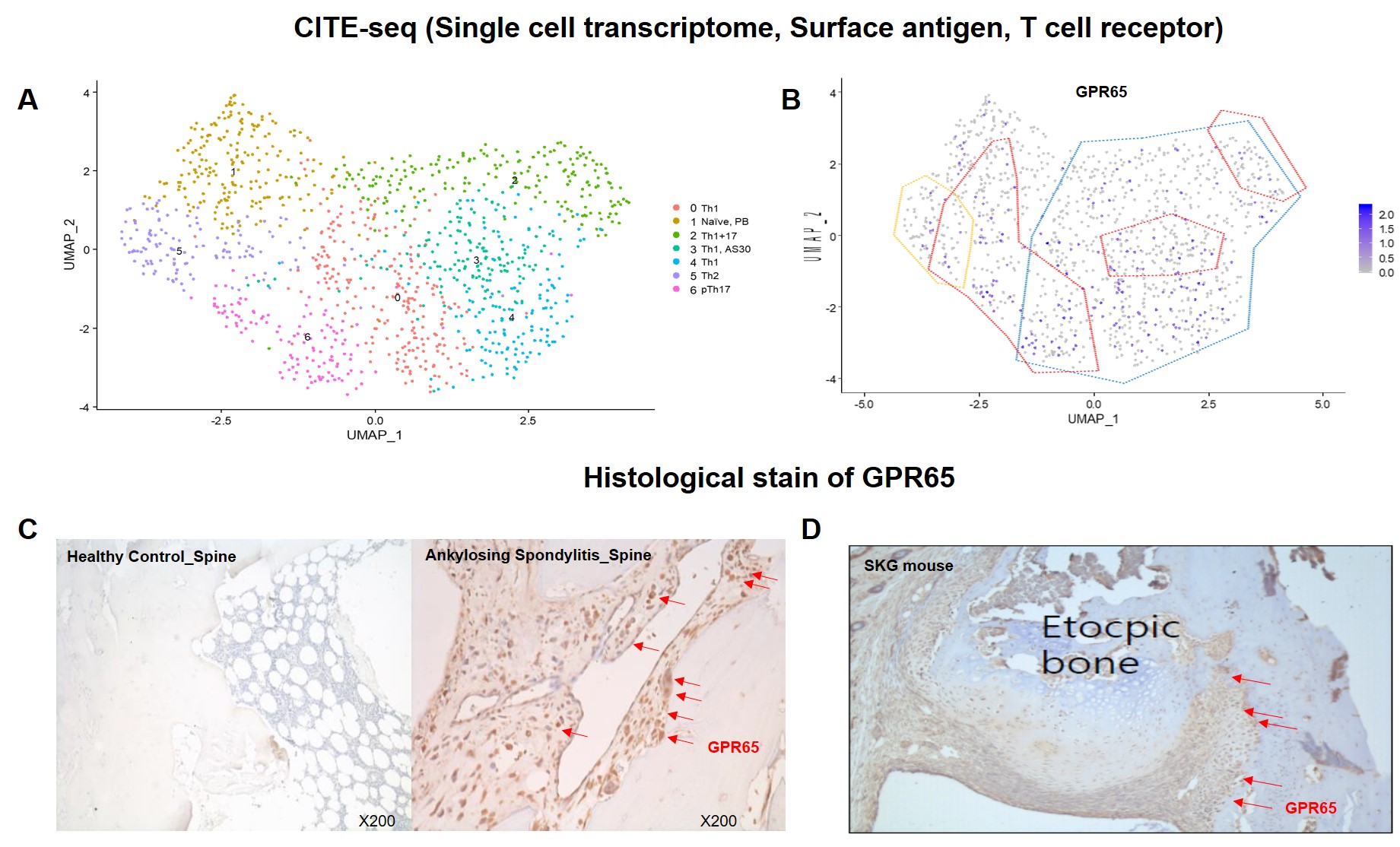
Previous research utilizing single-cell RNA sequencing (scRNA-seq) identified specific markers of pTh17 cells, among which GPR65 was notably upregulated (Figure 1A and 1B). This study aims to identify and validate GPR65 as a significantly upregulated molecule in pTh17 and spinal tissue in AS patients.
Methods: Using single-cell RNA sequencing (scRNA-seq) and surface protein expression analysis, we profiled blood and synovial cells from AS patients. Flow cytometry was used to measure the levels of IL-17, IFN-γ, and TNF-a in the PBMCs. Human spine tissue samples were obtained from both AS patients and healthy controls. These samples were used to observe the expression of GPR65. Additionally, SKG mice were used to model ectopic bone formation. Bones were excised from these mice to assess GPR65 expression through immunohistochemistry. Comparative analysis was conducted to assess the expression of GPR65 in the cells and spinal samples.
Results: Flow cytometry analysis demonstrated a noteworthy increase in the levels of IL-17, IFN-γ, and TNF-a in GPR65-positive cells compared to GPR65-negative cells. Additionally, the expression of GPR65 was found to be elevated in spinal tissue samples, as shown in Figure 1C. Furthermore, in the SKG mouse model, GPR65 was highly expressed at sites of ectopic new bone formation and was associated with severe inflammation, as depicted in Figure 1D. This finding suggests a critical role for GPR65 in the inflammatory processes and pathological bone formation observed in AS.
Conclusion: This study identifies GPR65 as a key molecule upregulated in pTh17 cells and spinal tissue in AS patients, correlating with increased IL-17, IFN-γ, and TNF-α levels. Elevated GPR65 expression in spinal tissues and ectopic bone formation sites in the SKG mouse model underscores its potential as a therapeutic target for managing AS.
To cite this abstract in AMA style:
Park Y, Park K, Jo S, Kim T, Kim T. Enhanced Expression of GPR65 in Inflammatory Sites and Bone Formation Regions in Ankylosing Spondylitis: Evidence from ScRNA-seq Analysis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/enhanced-expression-of-gpr65-in-inflammatory-sites-and-bone-formation-regions-in-ankylosing-spondylitis-evidence-from-scrna-seq-analysis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/enhanced-expression-of-gpr65-in-inflammatory-sites-and-bone-formation-regions-in-ankylosing-spondylitis-evidence-from-scrna-seq-analysis/