Session Information
Date: Sunday, November 17, 2024
Title: Systemic Sclerosis & Related Disorders – Basic Science Poster I
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: The precise molecular mechanisms driving fibrosis in diffuse cutaneous systemic sclerosis (dcSSc) remain to be elucidated. The immune regulatory programmed cell death protein 1 (PD-1) pathway is upregulated in inflammation and has been connected to fibrosis. In this study, we explore the impact of the PD-1 pathway in diffuse cutaneous systemic sclerosis (dcSSc), with a particular emphasis on immune activation and fibrogenesis.
Methods: We assessed PD-1 levels in plasma samples from 35 dcSSc patients and 20 healthy controls. We also analyzed PBMCs and dermal fibroblasts from dcSSc patients and healthy controls, and used recombinant PD-1 protein (PD-1:Fc), isotype controls, and anti-PD-1 antibodies to treat PBMCs, dermal fibroblast cultures, and co-cultures. Additionally, we sorted, bulk RNA sequenced, and analyzed the PD-1Hi and PD-1low populations of dcSSc PBMCs. Finally, we used a murine bleomycin model for lung injury to study the effect of PD-1:Fc on lung fibrosis in vivo.
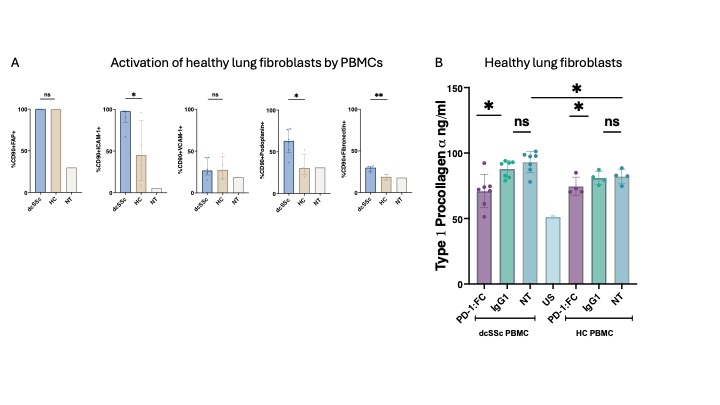
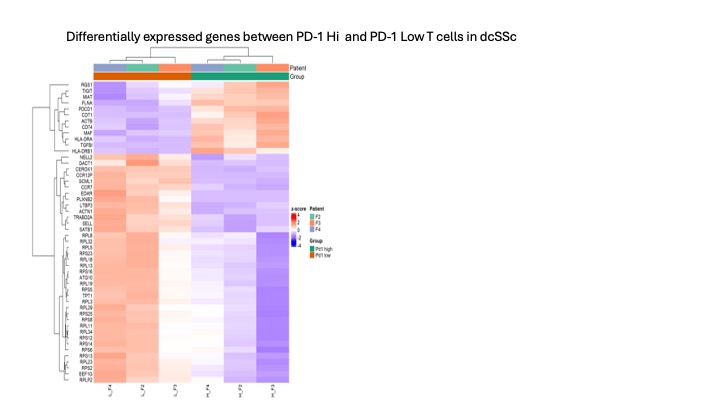
Results: PD-1 was elevated in dcSSc at both the soluble and cellular levels (p< 0.0001). Soluble PD-1 correlated with key clinical parameters such as CRP, FVC, and mRSS (all rho >0.3). Fibroblasts from dcSSc expressed high levels of PD-L1 and exhibited increased activation levels based on surface markers such as ICAM and VCAM. We identified a distinct myofibroblast population in dcSSc fibroblasts, displaying differential clustering compared to HCs fibroblasts. DcSSc fibroblasts produced increased amounts of extracellular matrix proteins (ECM) such as Type 1 Procollagen a (2-fold) and fibronectin (1.5-fold) when compared to HCs. This secretion was downregulated by PD-1:Fc (p=0.004 and p=0.01 respectively). Monocultures of dcSSc PBMCs and autologous dcSSc PBMCs and fibroblasts co-cultures were treated with PD-1:Fc, and we observed a significant decrease in inflammatory cytokines and ECM protein production compared to untreated samples (p< 0.01). Additionally, dcSSc PBMCs could activate dermal fibroblasts and healthy lung fibroblasts as evaluated by surface ICAM, fibronectin and podoplanin (Fig 1A) The addition of PD-1:Fc to these cultures significantly reduced Type 1 Procollagen a production (Fig 1B). RNA seq of sorted PD-1 hi vs PD-1 low expressing T cells revealed differential clustering and an exaggerated regulatory profile associated with the PD-1 hi T cell population (Fig 2).
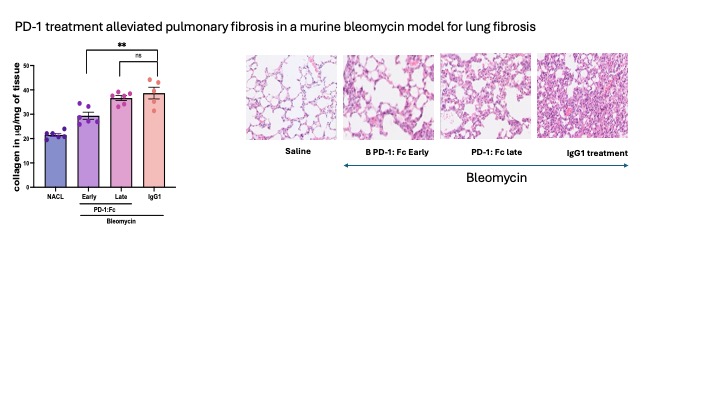
Our in vitro findings were strongly supported by animal studies in the bleomycin model of lung fibrosis. We demonstrated that early treatment with PD-1:Fc could inhibit the development of lung fibrosis and decrease the levels of fibrosisin the lungs (Fig 3). This effect was supported through the downregulation of inflammatory and profibrotic cytokines in plasma, among these IL-6 and TNF- (p< 0.01).
Conclusion: Our study underscores the significant role of the PD-1 pathway in dcSSc. Targeting the PD-1 pathway by PD-1:Fc attenuates fibrosis resulting from inflammation. These data suggest the PD-1 pathway as a potential treatment target to decrease the development of fibrosis in dcSSc.
To cite this abstract in AMA style:
Aspari M, Ong V, Soendergaard K, Naeser E, Hvid M, Tam A, Xu S, Denton C, Abraham D, Deleuran B, Greisen S. Engaging the PD-1 Pathway Attenuates Inflammation Associated Fibrosis in Systemic Sclerosis Fibroblasts and a Preclinical Mouse Model [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/engaging-the-pd-1-pathway-attenuates-inflammation-associated-fibrosis-in-systemic-sclerosis-fibroblasts-and-a-preclinical-mouse-model/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/engaging-the-pd-1-pathway-attenuates-inflammation-associated-fibrosis-in-systemic-sclerosis-fibroblasts-and-a-preclinical-mouse-model/