Session Information
Date: Monday, November 14, 2022
Title: Abstracts: T Cell Biology and Targets in Autoimmune and Inflammatory Disease
Session Type: Abstract Session
Session Time: 3:00PM-4:00PM
Background/Purpose: Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory condition with persistent unmet medical need despite significant treatment advances. The pathogenesis of RA is initiated by autoreactive effector T cells (Teffs) that are activated by autoantigens presented by human leukocyte antigens. This T cell reactivity breaks self-tolerance and triggers inflammatory responses against self-antigens, resulting in autoimmune disease. Autoimmunity is controlled by regulatory T cells (Tregs) in healthy individuals, but Treg activity can be deficient in patients with RA. Citrullinated proteins are created through post-translational modification by peptidylarginine deiminase enzymes and are abundant autoantigens at sites of inflammation. We have developed an autologous Treg cell therapy for patients that can target citrullinated proteins, with the aim to dampen inflammation and re-establish immune tolerance.
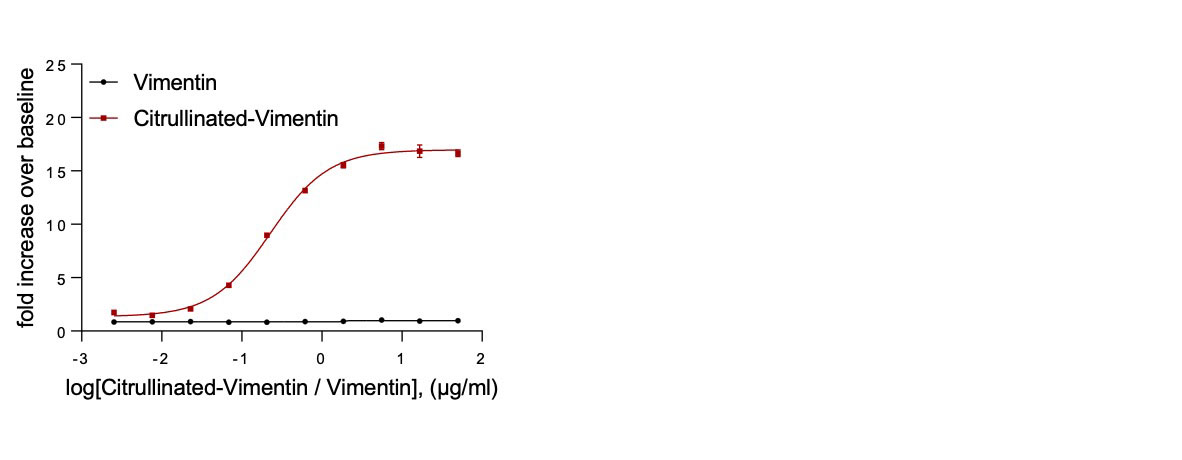
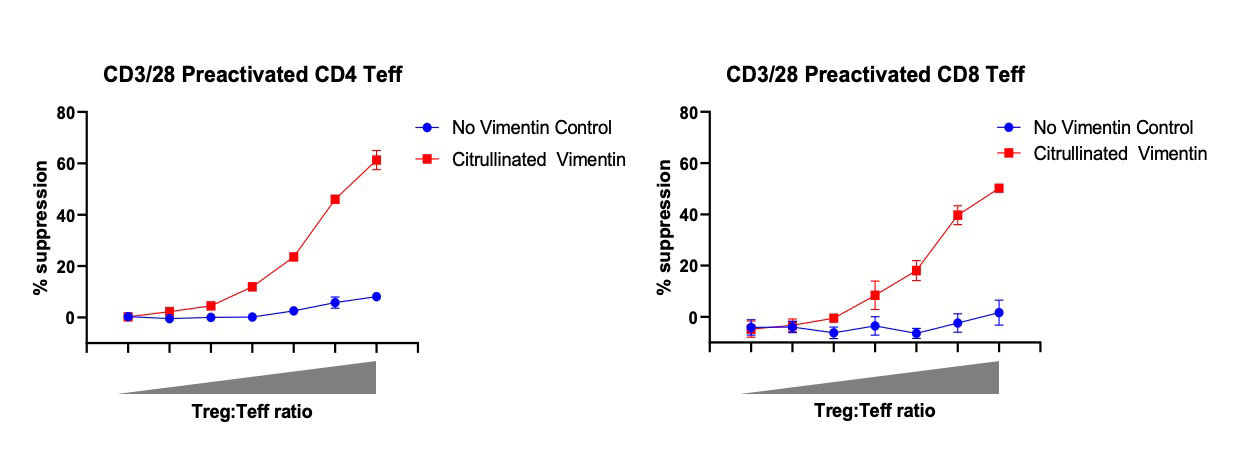
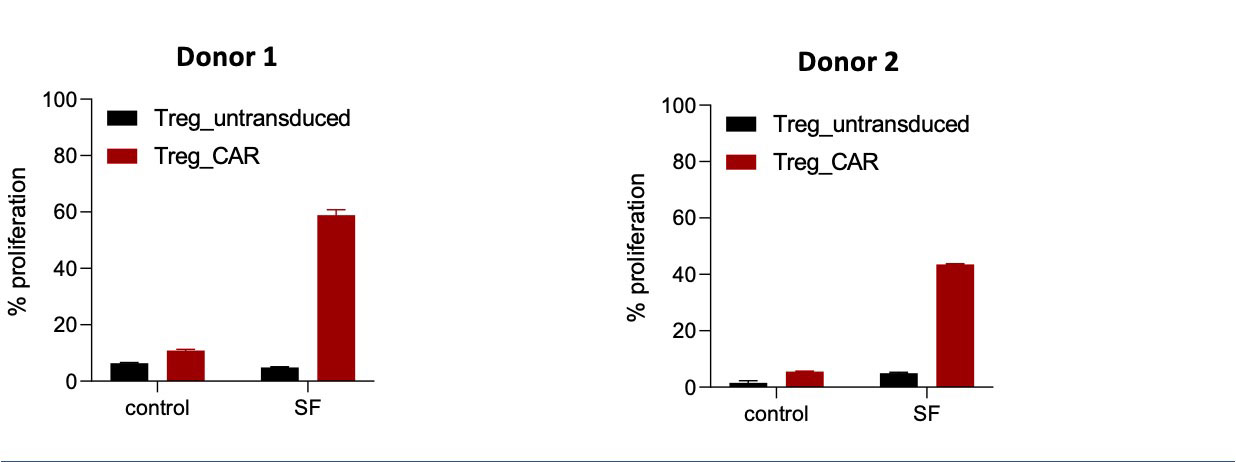
Methods: Tregs were engineered to express a chimeric antigen receptor (CAR) containing an extracellular binding domain specific for citrullinated proteins and an intracellular signaling domain to bypass the need for antigen presenting cell interaction. The specificity of the CAR Treg for its target was tested in vitro using citrullinated vimentin (CV), a citrullinated protein present in RA patient synovial fluid (SF), and its non-modified counterpart as a control. Assays were performed to analyze the ability of CV to activate CAR-expressing luciferase reporter cells, promote CAR Treg proliferation and augment the suppressive activity of the CAR Tregs. Lastly, the ability to activate the CAR Treg was evaluated using SF samples from RA patients.
Results: The CAR-expressing reporter cells were activated by CV but not by the unmodified protein, demonstrated by an increase in luciferase signal over background in an overnight in vitro assay (Figure 1). Activation of the CAR Tregs by bead bound citrullinated protein resulted in robust proliferation of the CAR Tregs and anti-inflammatory cytokine production (IL-10). The activated CAR Tregs suppressed the proliferation of both CD4 and CD8 Teffs in the presence of CV to a greater extent than Tregs not activated via CAR (Figure 2). CAR Tregs cocultured with SF from RA patients were activated in vitro and had a higher level of proliferation compared to untransduced controls, demonstrating that the cells can be activated by physiological antigens that are present within inflamed joints (Figure 3).
Conclusion: CAR Tregs specific for citrullinated proteins present in the inflamed joints of RA patients can be manufactured, are activated by antigen, and suppress the activity of Teff cells. Treatment with these CAR Treg cells may be able to reduce inflammation and restore immune tolerance in patients with RA.
To cite this abstract in AMA style:
van der Vuurst de Vries A, Hooper K, Graf J, Tuckwell K, Beilke J. Development of a Novel Regulatory T Cell-Based Therapy for Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/development-of-a-novel-regulatory-t-cell-based-therapy-for-patients-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-a-novel-regulatory-t-cell-based-therapy-for-patients-with-rheumatoid-arthritis/