Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: Detailed characterization of the plasma proteome may provide insights into the dynamic molecular changes preceding gout. A previous cross-sectional study of pre-existing gout (n=330 gout patients and 7 candidate proteins)1 reported higher CXCL8 levels among prevalent gout vs non-gout, but data on pre-diagnostic proteomic biomarkers are sparse.
We investigated the relation between large-scale pre-diagnostic proteomics and risk of incident gout in a nationwide prospective cohort, accounting for serum urate levels, and explored biological mechanisms connected to the gout-associated proteins and upstream factors.
Methods: We included 48,898 UK Biobank participants with proteomic measures and no history of gout or urate-lowering therapy use at baseline (mean age 56.7 years, 45% males). Proteomic measures (n=2923, via Olink Explore 3072) were quantified from baseline blood samples via Olink’s Proximity Extension Assay technology.2
Multivariable Cox models were used to assess association between protein levels and risk of incident gout (from linked primary care and inpatient diagnoses), before and after serum urate adjustment, using false discovery rate (PFDR) of < 0.05. We performed two-sample Mendelian randomization (MR) to explore potential causal roles of the statistically significant proteins, leveraging genetic association data for loci mapping within 1Mb of the protein-encoding gene controlling expression in cis,2 and from the new GlobalGout GWAS.
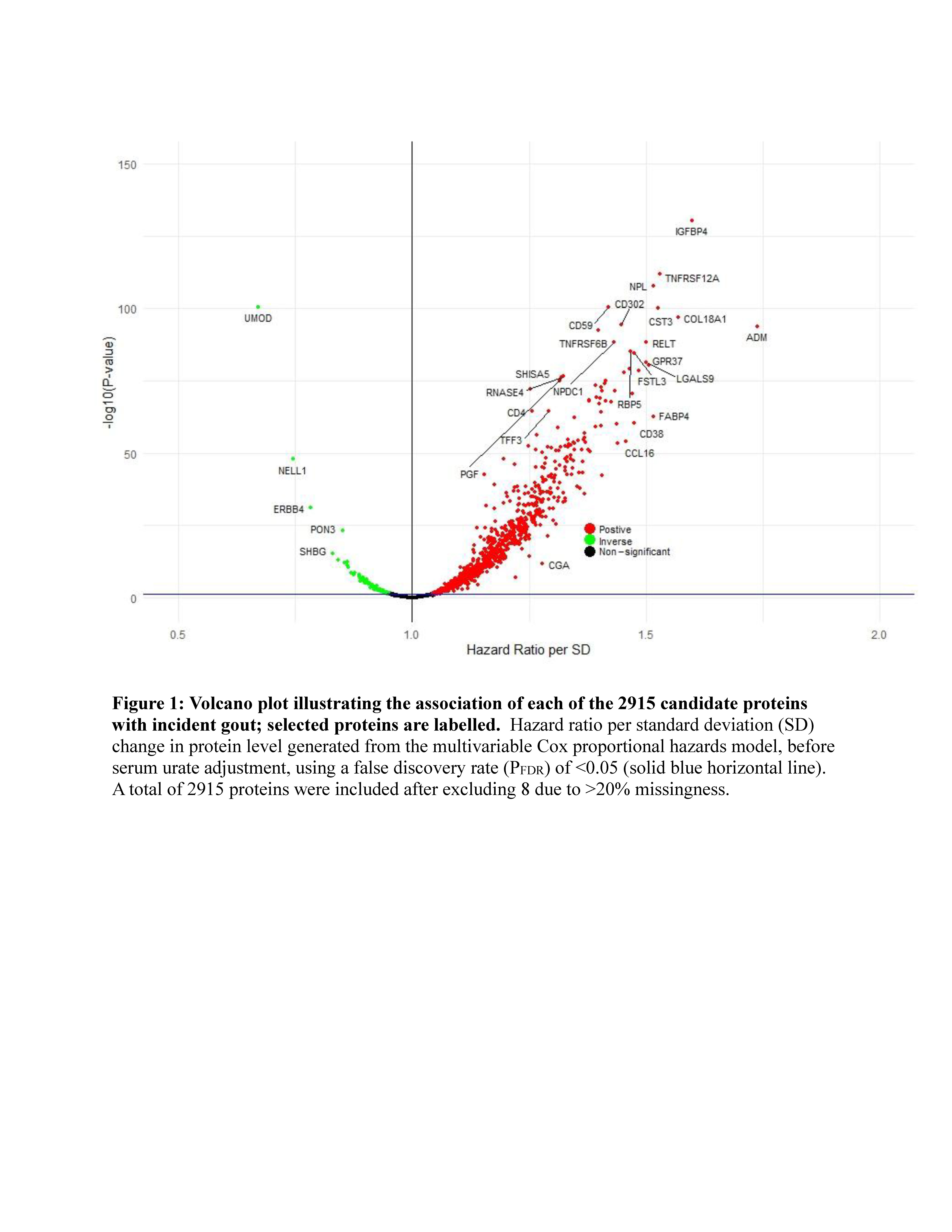
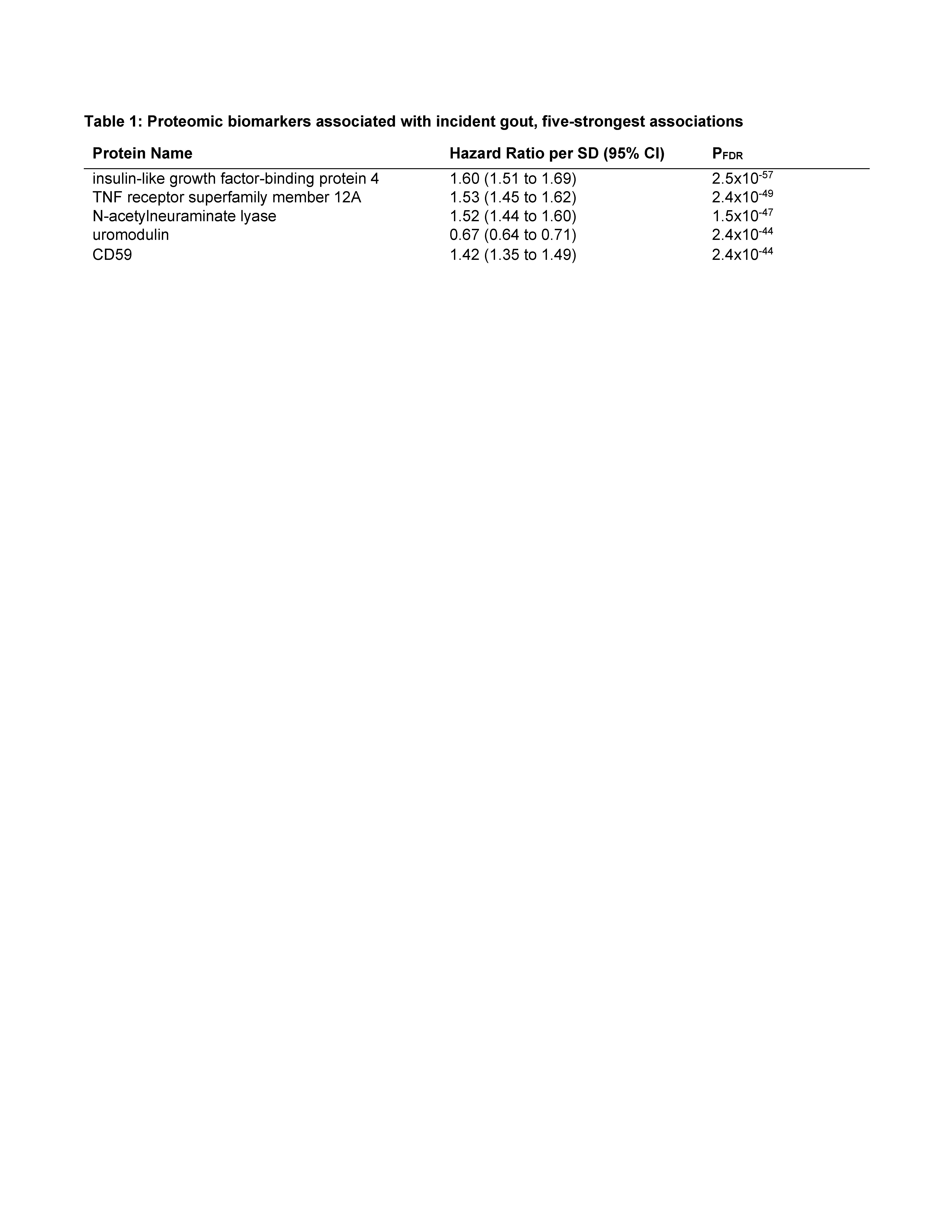
Results: There were 1095 incident gout cases over mean follow-up of 13.2 years. Levels of 1027 proteins were associated with risk of incident gout before urate adjustment (Figure 1), with the strongest associations (Table 1) including insulin-like growth factor-binding protein 4 (hazard ratio [HR] 1.60, 95% CI 1.51-1.69 per SD; PFDR 2.5×10-57) and uromodulin (0.67, 0.64-0.71; PFDR 2.4×10-44). Other key associations were CD38, a proinflammatory enzyme expressed in macrophages, CCL16, a substantial monocyte chemoattractant, and ADM, which regulates inflammation, including in the synovium. 41 proteins remained associated with risk of incident gout after serum urate adjustment, including uromodulin (HR 0.88, 0.83-0.94; PFDR=0.01)
MR findings supported causal roles for 72 of the 817 measurable proteins associated with gout before urate adjustment, and 3/37 measurable proteins after urate adjustment (Secretagogin, Spondin-1 and TNF receptor superfamily member 6B).
Top biological pathways identified via the Ingenuity Pathway Analysis software were pathogen induced cytokine storm signalling (PFDR=3.2×10-48), hepatic fibrosis (PFDR=1.3×10-47), and neutrophil degranulation (PFDR=1.3×10-45). Those involved in wound healing signalling, regulation of insulin-like growth factor transport and uptake, and granulocyte adhesion and diapedesis were also among the top overrepresented biological pathways.
TNF was identified as the top upstream regulator of gout-associated proteins; others included IL-1B and IL-4 cytokines.
Conclusion: These prospective findings provide insight on pathophysiological mechanisms leading to gout, including after hyperuricemia, and prioritize protein-gout genetic associations for future research.
To cite this abstract in AMA style:
McCormick N, Joshi A, Terkeltaub R, Merriman T, Nayor M, Yokose C, Choi H. Circulating Proteomic Profiles and Incident Gout Risk: Prospective Cohort Study of >48,000 Men and Women [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/circulating-proteomic-profiles-and-incident-gout-risk-prospective-cohort-study-of-48000-men-and-women/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/circulating-proteomic-profiles-and-incident-gout-risk-prospective-cohort-study-of-48000-men-and-women/