Session Information
Date: Saturday, November 7, 2020
Title: SLE – Treatment Poster I
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Incomplete B-cell and plasmablast depletion, as measured using highly sensitive flow cytometry (HSFC), is associated with lower response rates following rituximab in SLE [1]. Enhanced B-cell depletion with the type II anti-CD20 monoclonal antibody obinutuzumab resulted in increased renal responses in proliferative lupus nephritis (LN) in the NOBILITY trial (NCT02550652) and will be further evaluated in the Phase 3 REGENCY trial (NCT04221477). The objective of this analysis was to measure peripheral B-cells, B-cell subsets (naïve, memory and plasmablast) and B-cell activating factor (BAFF) levels and to assess associations between B-cell depletion and renal response in LN patients in a clinical trial of obinutuzumab.
Methods: 126 patients with active Class III/IV LN were randomized to obinutuzumab or placebo infusions in combination with mycophenolate and glucocorticoids. Peripheral B-cells were measured using a HSFC method with a lower limit of quantitation of 0.441 cells/μL. Serum levels of BAFF were evaluated using ELISA. Sustained depletion was defined by total B-cells below the limit of detection at both weeks 24 and 52. Renal response definitions from Phase 2 NOBILITY and Phase 3 REGENCY trials were used.
Results: Obinutuzumab resulted in rapid depletion of total B-cells, memory and naïve B-cells, and plasmablasts from peripheral blood, with 88% of obinutuzumab patients depleted to < 0.441 total B-cells/μL at week 2 (Figure). Mean serum BAFF increased from 4,585 pg/mL at baseline to 14,601 pg/mL at week 52 in the obinutuzumab group. Sustained B-cell depletion was achieved in 32/52 (62%) of patients with complete data and was associated with a higher renal response rate at week 76 (Table), although patients who achieved sustained depletion also had lower baseline proteinuria and serum creatinine.
Conclusion: Obinutuzumab, a type II anti-CD20 mAb, mediated rapid, complete and sustained depletion of peripheral B-cells and plasmablasts and large increases in serum BAFF. Similar to previous reports, sustained B-cell depletion was associated with increased renal response though there may have been confounding factors. REGENCY is being conducted to further evaluate the therapeutic hypothesis with obinutuzumab in LN.
References:
- Md Yusof MY et al. Ann Rheum Dis. 2017;76:1829-36.
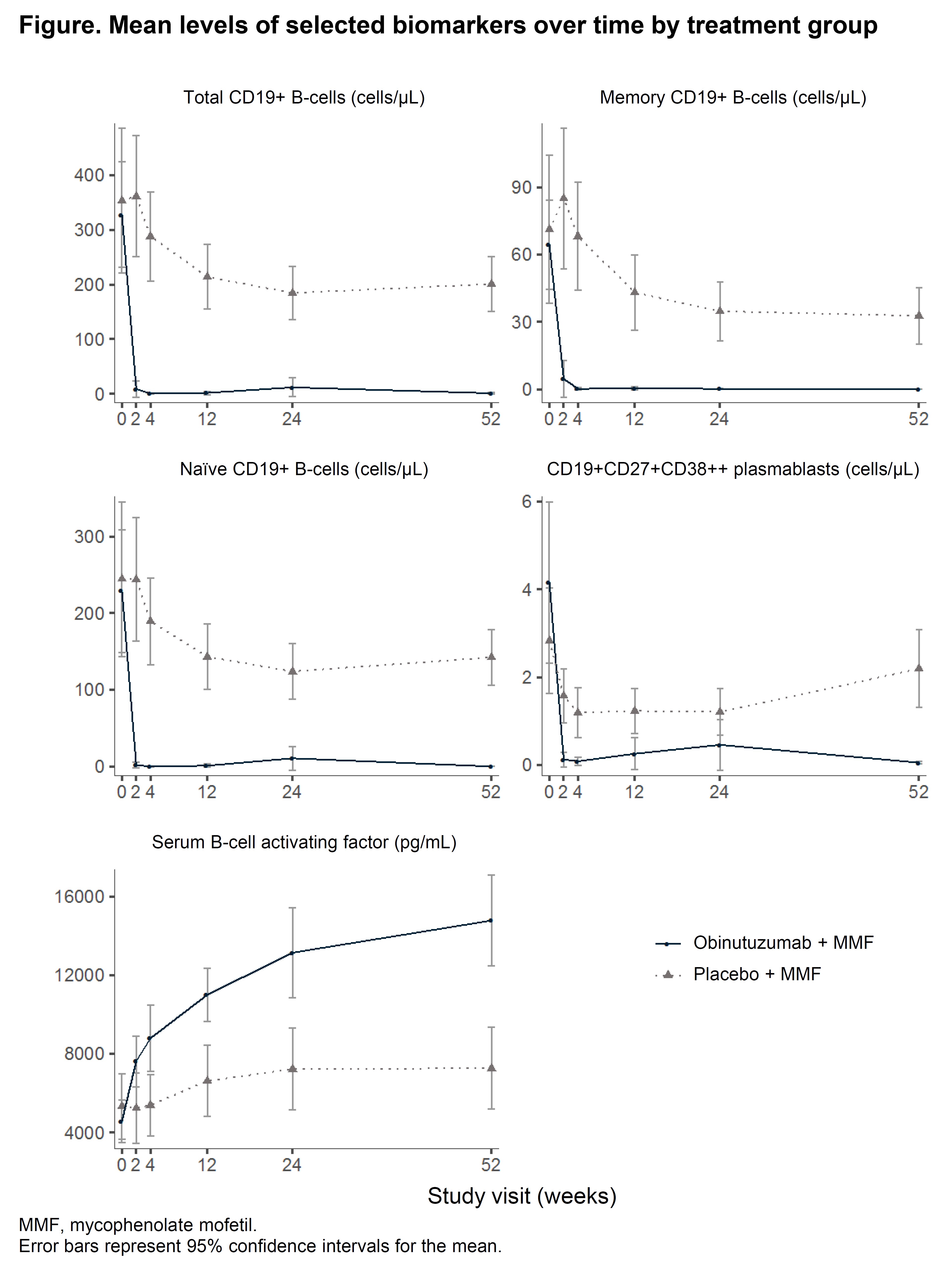 Figure. Mean levels of selected biomarkers over time by treatment group.
Figure. Mean levels of selected biomarkers over time by treatment group.
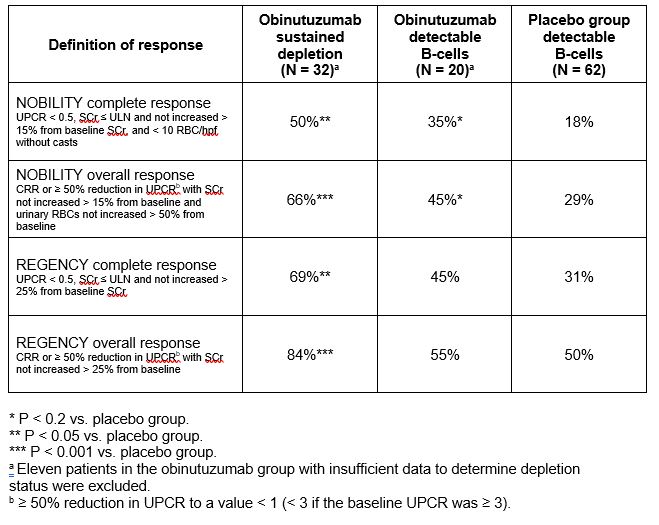 Table. Data from NOBILITY at week 76 by depletion status at weeks 24 and 52.
Table. Data from NOBILITY at week 76 by depletion status at weeks 24 and 52.
To cite this abstract in AMA style:
Vital E, Remy P, Quintana Porras L, Chiche L, Chauveau D, Furie R, Schindler T, Garg J, Cascino M, Amoura Z, Doria A, Looney C, Roccatello D. Biomarkers of B-cell Depletion and Response in a Randomized, Controlled Trial of Obinutuzumab for Proliferative Lupus Nephritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/biomarkers-of-b-cell-depletion-and-response-in-a-randomized-controlled-trial-of-obinutuzumab-for-proliferative-lupus-nephritis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/biomarkers-of-b-cell-depletion-and-response-in-a-randomized-controlled-trial-of-obinutuzumab-for-proliferative-lupus-nephritis/
