Session Information
Date: Wednesday, October 29, 2025
Title: Abstracts: Systemic Sclerosis & Related Disorders – Clinical III (2651–2656)
Session Type: Abstract Session
Session Time: 9:45AM-10:00AM
Background/Purpose: Most patients with systemic sclerosis (SSc) experience gastrointestinal (GI) dysmotility. The enteric nervous system (ENS) regulates GI motility, and its dysfunction causes dysmotility. A subset of SSc patients harbor autoantibodies against the M2 mitochondrial antigen (AM2A). Here, we investigate whether M2 is expressed by specific ENS cells, and if AM2A associate with GI dysmotility in SSc patients.
Methods: Patients with SSc were prospectively enrolled from a large Scleroderma Center into the NIH-funded cohort based on ACR/EULAR criteria or CREST features and GI symptomatology. Clinical phenotyping, including Medsger Severity Scores, autoantibody profiles, and overlap diagnoses (e.g., PBC), was performed at enrollment and during routine follow-up. Whole gut transit (WGT) scintigraphy was conducted using radiolabeled meals to evaluate GI motility. AM2A autoantibodies were assessed by ELISA, and their uptake was studied via immunostaining in HepG2 cells, human intestinal tissue, and neural-crest lineage mouse models using confocal microscopy. Functional effects of AM2A were tested with Seahorse assays. Immunoprecipitation and Western blot confirmed antibody-protein interactions. All protocols were IRB-approved, and statistical analyses included t-tests, chi-square, regression, and Spearman correlation, with significance set at p< 0.05.
Results: Nineteen of 147 patients (12.9%) were AM2A positive. AM2A positivity was significantly associated with slower transit in the esophagus (β -14.4, 95%CI -26.2, -2.6) and stomach (β -7.9, 95% CI -14.1, -1.6). Immunostaining demonstrated pan-mitochondrial antigens TOM-20 and M2 enrichment in human ENS neurons, specifically in mesoderm-derived enteric neurons (MENS). Autoantibodies in SSc sera penetrated live adult murine MENs and HepG2 cells, when adult murine longitudinal muscle containing myenteric plexus (LM-MP) tissue and HepG2 cells were cultured with SSc sera. Upon penetrating live cells, AM2A localized to mitochondria, and immunoprecipitation demonstrated binding to the M2 antigen. Seahorse assays show that penetration of HepG2 cells with SSc-AM2A altered cellular respiration suggesting that penetrating AM2A are pathogenic.
Conclusion: AM2A in SSc patients associate with slower GI transit. SSc autoantibodies penetrate live cells in vitro, and SSc-AM2A penetrate live cells to target the mitochondrial M2 antigen and cause functional deficits in cellular respiration. Since a subset of enteric neurons (MENs) is enriched in mitochondria, which are similarly penetrated by SSc-AM2A ex vivo, the presence of GI dysmotility in SSc patients harboring AM2A suggests that SSc-AM2A may penetrate MENs in vivo, driving ENS and GI dysfunction. Further studies are warranted to test how AM2A alter ENS function in vivo to contribute to GI dysmotility in SSc.
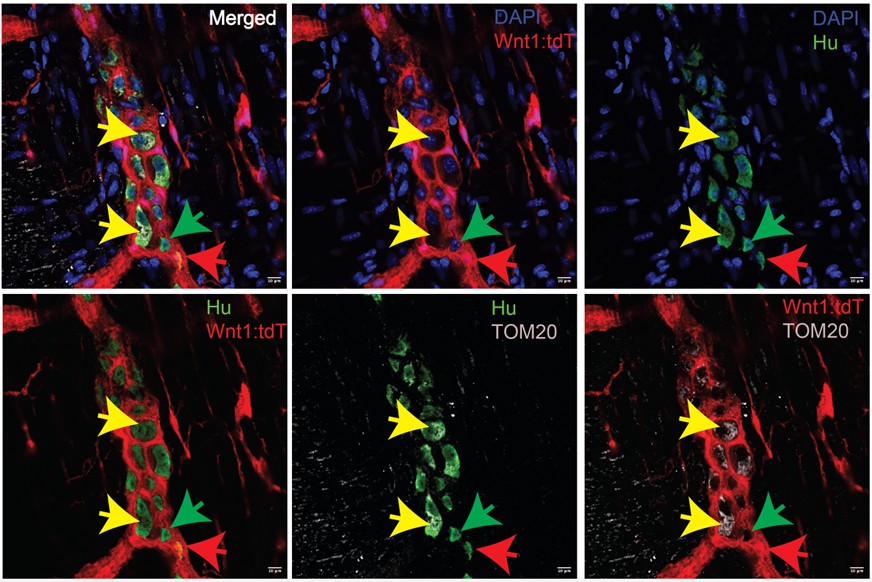 Figure 1. Figure 3: A subset of mesoderm-derived enteric neurons (MENs) are enriched in mitochondria. Immunostaining of adult murine small intestinal longitudinal muscle – myenteric plexus (LM-MP) tissue with ANNA1 antisera containing antibodies against Hu (green) and antibodies against the pan-mitochondrial antigen TOM20 (cyan). LM-MP is derived from an neural crest lineage-specific adult mouse Wnt1-cre:tdTomato, where tdTomato (red) labels neural crest-derived cells. The representative image shows that tdTomato- Hu+ neurons (MENs, yellow arrows) are enriched in TOM20, while the neural crest-derived neurons (NENs, red arrow) and a subset of MENs (green arrow) are not. Nuclei are labeled with DAPI (blue). Scale bar = 10 m.
Figure 1. Figure 3: A subset of mesoderm-derived enteric neurons (MENs) are enriched in mitochondria. Immunostaining of adult murine small intestinal longitudinal muscle – myenteric plexus (LM-MP) tissue with ANNA1 antisera containing antibodies against Hu (green) and antibodies against the pan-mitochondrial antigen TOM20 (cyan). LM-MP is derived from an neural crest lineage-specific adult mouse Wnt1-cre:tdTomato, where tdTomato (red) labels neural crest-derived cells. The representative image shows that tdTomato- Hu+ neurons (MENs, yellow arrows) are enriched in TOM20, while the neural crest-derived neurons (NENs, red arrow) and a subset of MENs (green arrow) are not. Nuclei are labeled with DAPI (blue). Scale bar = 10 m.
.jpg) Figure 2. SSc-AM2A penetrate viable MENs ex vivo. Adult murine small intestinal LM-MP tissues from the Wnt1-cre:tdTomato lineage fate mapping mouse when cultured with SSc patient sera containing AM2A (FW-2530), and immunostained with commercially available anti-DLAT antibodies post-culture show that tdTomato-negative myenteric neurons or MENs show presence of SSc autoantibodies (AM2A; cyan) within their soma which co-localizes with the presence of DLAT (green) immunostaining (white arrows). Nuclei are labeled with DAPI (blue). Scale bar = 10 m.
Figure 2. SSc-AM2A penetrate viable MENs ex vivo. Adult murine small intestinal LM-MP tissues from the Wnt1-cre:tdTomato lineage fate mapping mouse when cultured with SSc patient sera containing AM2A (FW-2530), and immunostained with commercially available anti-DLAT antibodies post-culture show that tdTomato-negative myenteric neurons or MENs show presence of SSc autoantibodies (AM2A; cyan) within their soma which co-localizes with the presence of DLAT (green) immunostaining (white arrows). Nuclei are labeled with DAPI (blue). Scale bar = 10 m.
.jpg) Figure 3. SSc autoantibodies penetrate viable cells and engage intracellular targets upon uptake. HepG2 cells when cultured with and without SSc antisera (red) can be used to test intracellular uptake. Live HepG2 cells cultured (A) without SSc antisera, and with (B) AM2A+ SSc antisera (patient FW-2530), and (C) AM2A- SSc antisera (patient FW-2340), were subsequently fixed and immunostained with commercially available anti-M2/PDC-E2 antibody (green) and imaged to observe that SSc autoantibodies were present within cells cultured with SSc patient antisera. Furthermore, AM2A+ antisera was detected in PDC-E2- expressing mitochondria (B, white arrow), but the AM2A- antisera labeled cellular compartments other than the PDC-E2-expressing mitochondria (C, white arrows). Nuclei are labeled with DAPI (blue). Scale bar = 10 μm.
Figure 3. SSc autoantibodies penetrate viable cells and engage intracellular targets upon uptake. HepG2 cells when cultured with and without SSc antisera (red) can be used to test intracellular uptake. Live HepG2 cells cultured (A) without SSc antisera, and with (B) AM2A+ SSc antisera (patient FW-2530), and (C) AM2A- SSc antisera (patient FW-2340), were subsequently fixed and immunostained with commercially available anti-M2/PDC-E2 antibody (green) and imaged to observe that SSc autoantibodies were present within cells cultured with SSc patient antisera. Furthermore, AM2A+ antisera was detected in PDC-E2- expressing mitochondria (B, white arrow), but the AM2A- antisera labeled cellular compartments other than the PDC-E2-expressing mitochondria (C, white arrows). Nuclei are labeled with DAPI (blue). Scale bar = 10 μm.
To cite this abstract in AMA style:
McMahan Z, Puttapaka S, Casciola-Rosen L, Kaniecki T, Gutierrez L, Hong MIng S, Seika P, Kulkarni S. Anti-mitochondrial antibodies in systemic sclerosis target enteric neurons and are associated with GI dysmotility [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/anti-mitochondrial-antibodies-in-systemic-sclerosis-target-enteric-neurons-and-are-associated-with-gi-dysmotility/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/anti-mitochondrial-antibodies-in-systemic-sclerosis-target-enteric-neurons-and-are-associated-with-gi-dysmotility/
