Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Unknown factors trigger the transition of anti-Ro+ mothers of neonatal lupus (NL) children from preclinical autoimmunity to clinical disease. One candidate may be the gut microbiome with specific taxa serving as environmental triggers contributing to clinical phenotype. We have previously reported differential abundances between anti-Ro+ mothers versus healthy controls (HC), noting a bloom in relative abundance of protective commensals including the genus Bacteroides. This taxon promotes tolerance at the interface of mucosal immunity. This study was initiated to test for associations of abundance of Bacteroides, and disease groups and for associations of anti-Ro titers.
Methods: Subjects included 125 RRNL mothers and 23 healthy controls. Stool microbiome of anti-Ro+ women in RRNL (asymptomatic and undifferentiated autoimmune syndrome (Asym/UAS), and SLE, SS, SS/SLE (SS/SLE)), and healthy controls (HC) were processed using 16S ribosomal RNA sequencing and use of a standard informatics pipeline. Tests for differential relative abundances of stool taxa applied compositional data analysis methods, linear models and adjustments for multiple comparisons. Assessments also included titers and specificities of anti-Ro using ELISA.
Results: The relative abundances varied as a function of clinical severity (HC < Asym/UAS < SS/SLE) shown across 13 genera (FDR < 0.05) including Bacteroides. Importantly, these ordered differences were maintained through the taxonomic hierarchy to Bacteroides (Figure 1) with the exception at species level Bacteroides vulgatus whereby relative median abundance reported at HC < SS/SLE < Asym/UAS (Figure 2), a finding that supported a focus on unique features of Asym/UAS that relate to Bacteroides. The initial analysis involved a comparison of Asym/UAS relative to HC. Applying multiple test correction, we observed an association of Bacteroidetes (P< 0.0001), as well as taxa at subsequent lower taxonomic levels including Bacteroides (P= 2.45 x 10-7), reflecting a higher relative abundance in Asym/UAS relative to HC. A subsequent analysis involved associations of Bacteroides relating to anti-Ro autoantibodies. While high titers of anti-Ro60 IgG and IgA as well as anti-Ro52 IgG and IgA titers were present in all NL mothers and absent in HC, there was an association between anti-Ro and Bacteroides relative abundance with an observed genus-by-disease interaction (P=0.0045; β=0.43 SE(β)=0.15) for Ro52 IgA levels. Specifically, with anti-Ro as an outcome, levels were relatively constant for individuals diagnosed with SS/SLE but decreased with abundance in Asym/UAS (P< 0.005, Figure 3). These data suggest that the abundance of Bacteroides may influence features that are a distinction of SS/SLE such as high levels of autoantibody, which in this study was favored by low abundance of Bacteroides.
Conclusion: The data provide intriguing initial evidence that a permissive factor in NL anti-Ro+ mothers which links overt SLE immunity to a gut commensal, Bacteroides vulgatus.
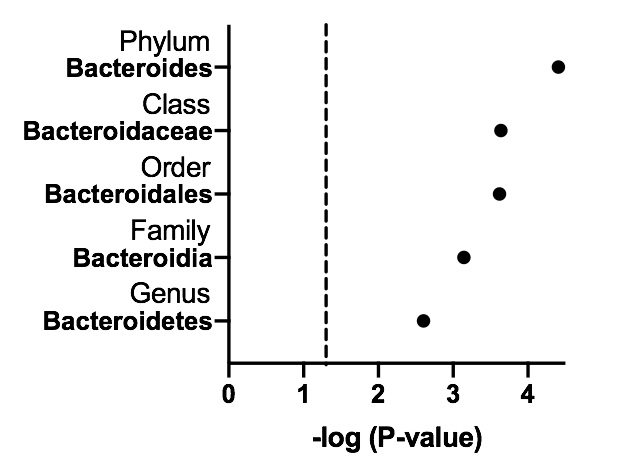 Figure1_RoAb.jpeg”Figure 1. Analysis of the stool genus, Bacteroides, meeting statistical significance (FDR P-value < 0.05) using the taxonomic stepdown method
Figure1_RoAb.jpeg”Figure 1. Analysis of the stool genus, Bacteroides, meeting statistical significance (FDR P-value < 0.05) using the taxonomic stepdown method
 Figure2_RoAb.jpeg”Figure 2. Species does not share ordinal significance, a feature that was evident at family and genus levels.
Figure2_RoAb.jpeg”Figure 2. Species does not share ordinal significance, a feature that was evident at family and genus levels.
 Figure 3_autoabs.jpeg”Figure 3. With anti-Ro52 IgA as an outcome, levels of autoantibody decreased with abundance in Asym/UAS subjects (P < 0.005).
Figure 3_autoabs.jpeg”Figure 3. With anti-Ro52 IgA as an outcome, levels of autoantibody decreased with abundance in Asym/UAS subjects (P < 0.005).
To cite this abstract in AMA style:
Clancy R, Marion M, Ainsworth H, Chang M, Howard T, Izmirly P, Masson M, Buyon J, Langefeld C. A Permissive Factor of Anti-Ro+ Mothers of Neonatal Lupus Children Is Linked to Overt SLE Associated with Immunity to a Gut Commensal [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/a-permissive-factor-of-anti-ro-mothers-of-neonatal-lupus-children-is-linked-to-overt-sle-associated-with-immunity-to-a-gut-commensal/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-permissive-factor-of-anti-ro-mothers-of-neonatal-lupus-children-is-linked-to-overt-sle-associated-with-immunity-to-a-gut-commensal/
