Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: A central feature of osteoarthritis (OA) is progressive cartilage degradation driven by increased production of matrix degrading enzymes including MMP-13. There is a critical need for a therapeutic that halts the loss of joint tissue in OA. Secretion of MMPs by chondrocytes can be triggered by extracellular matrix fragments, such as fibronectin fragments (FN-fs), or by inflammatory cytokines secreted by synovial fibroblasts, such as IL-6. Using RNAseq analysis, we have shown that treating normal chondrocytes with a recombinant FN-f (FN7-10) induces an OA chondrocyte phenotype. The goal of this project was to develop and test a high-throughput screen (HTS) using primary human chondrocytes treated with FN7-10 to identify non-cytotoxic compounds that inhibit the catabolic signaling responsible for production of MMPs and proinflammatory mediators.
Methods: We used FN7-10 as an OA relevant catabolic stimulus and MMP-13 as the readout. Primary human chondrocytes were isolated from cadaver donor ankle tissue, plated on 384-well pre-stamped compound plates, and then treated with recombinant FN7-10. For a control, we included lorecivivint, a known inhibitor of chondrocyte MMP production currently in clinical trials. After 24 hours, a viability assay was performed using calcein AM fluorescence staining. Next, APMA was added to activate MMPs secreted into the cell culture medium, and an MMP-13 responsive fluorogenic probe was used to detect active MMP-13 using a microplate reader. Secondary testing included measuring MMP-13 and IL-6 production by immunoblotting conditioned media from chondrocytes and OA synovial fibroblasts.
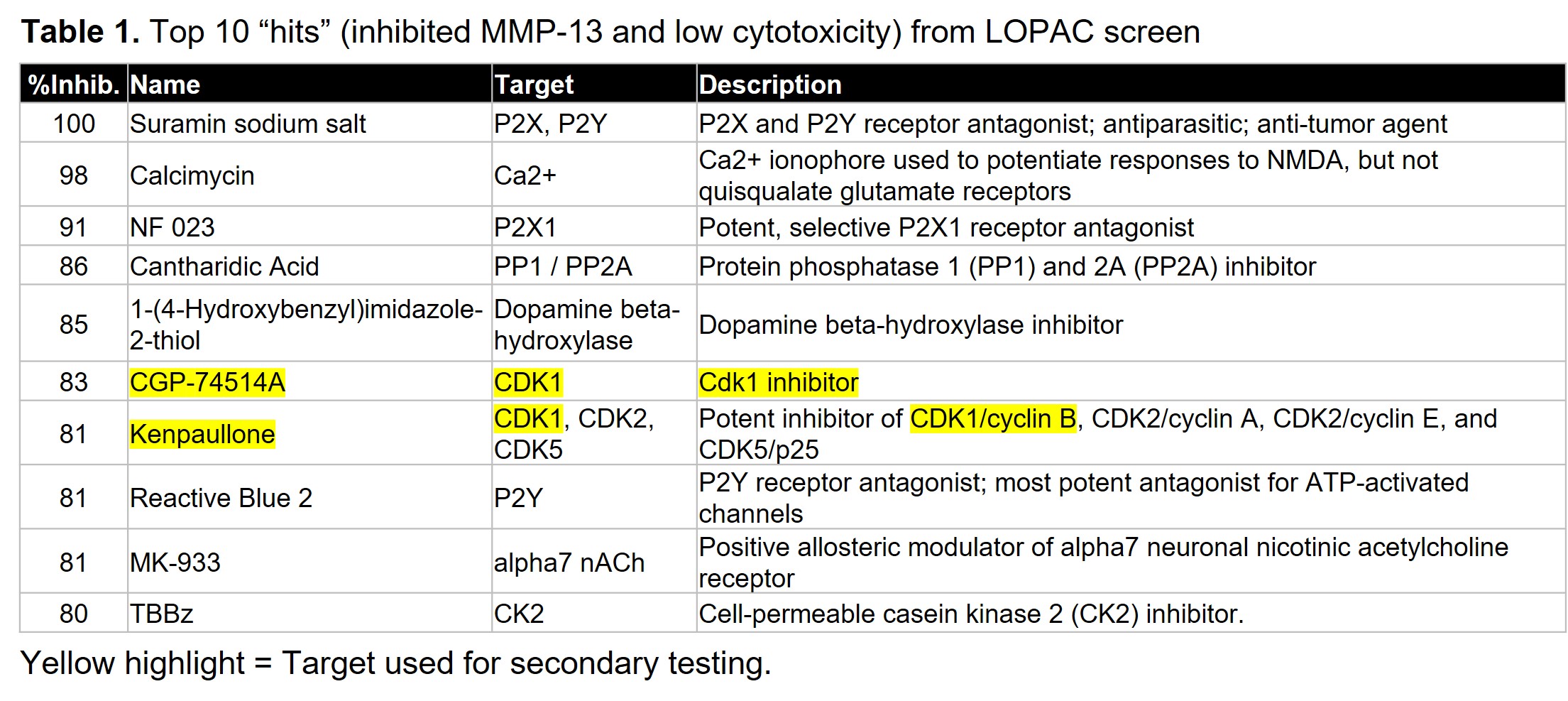
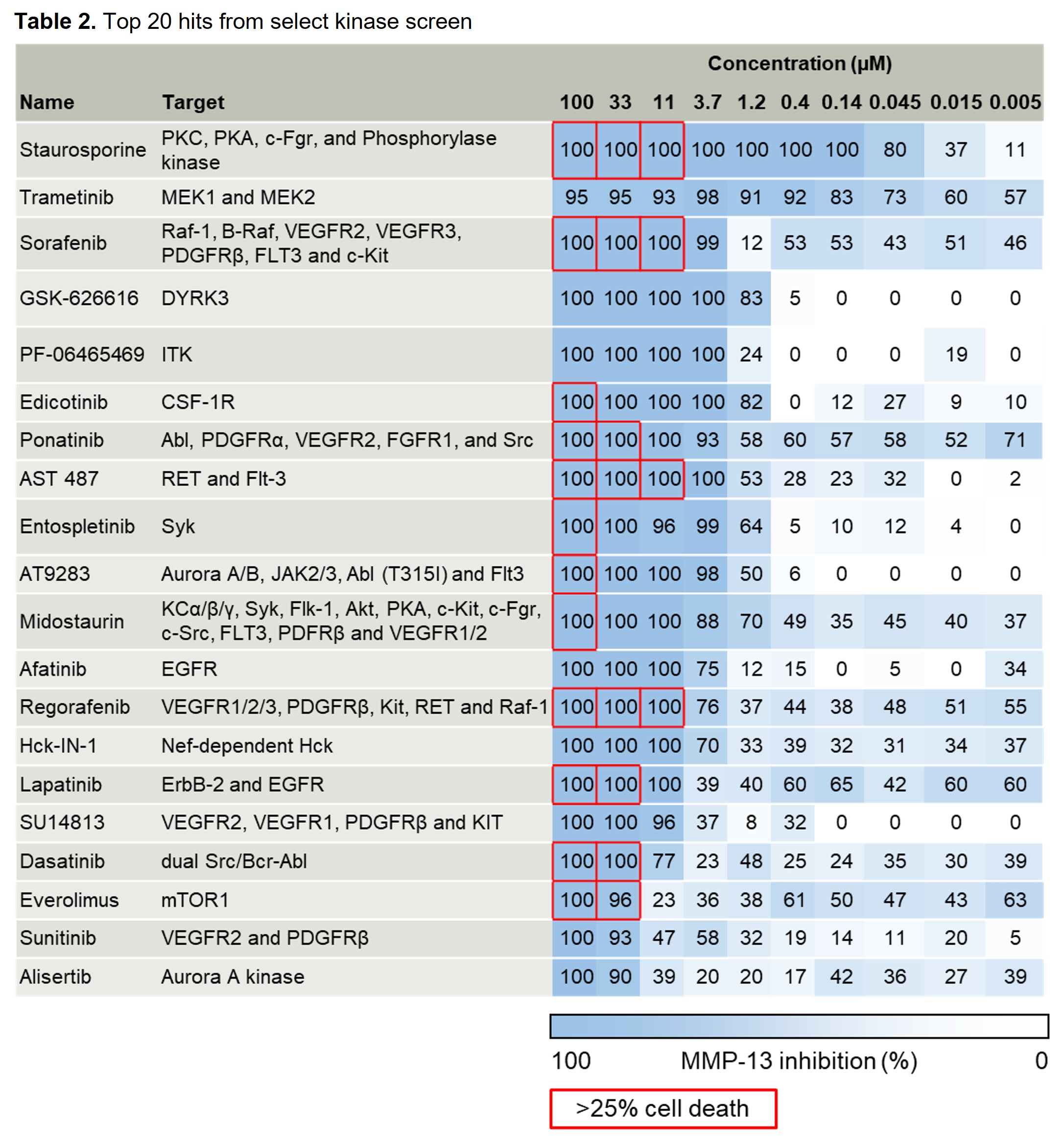
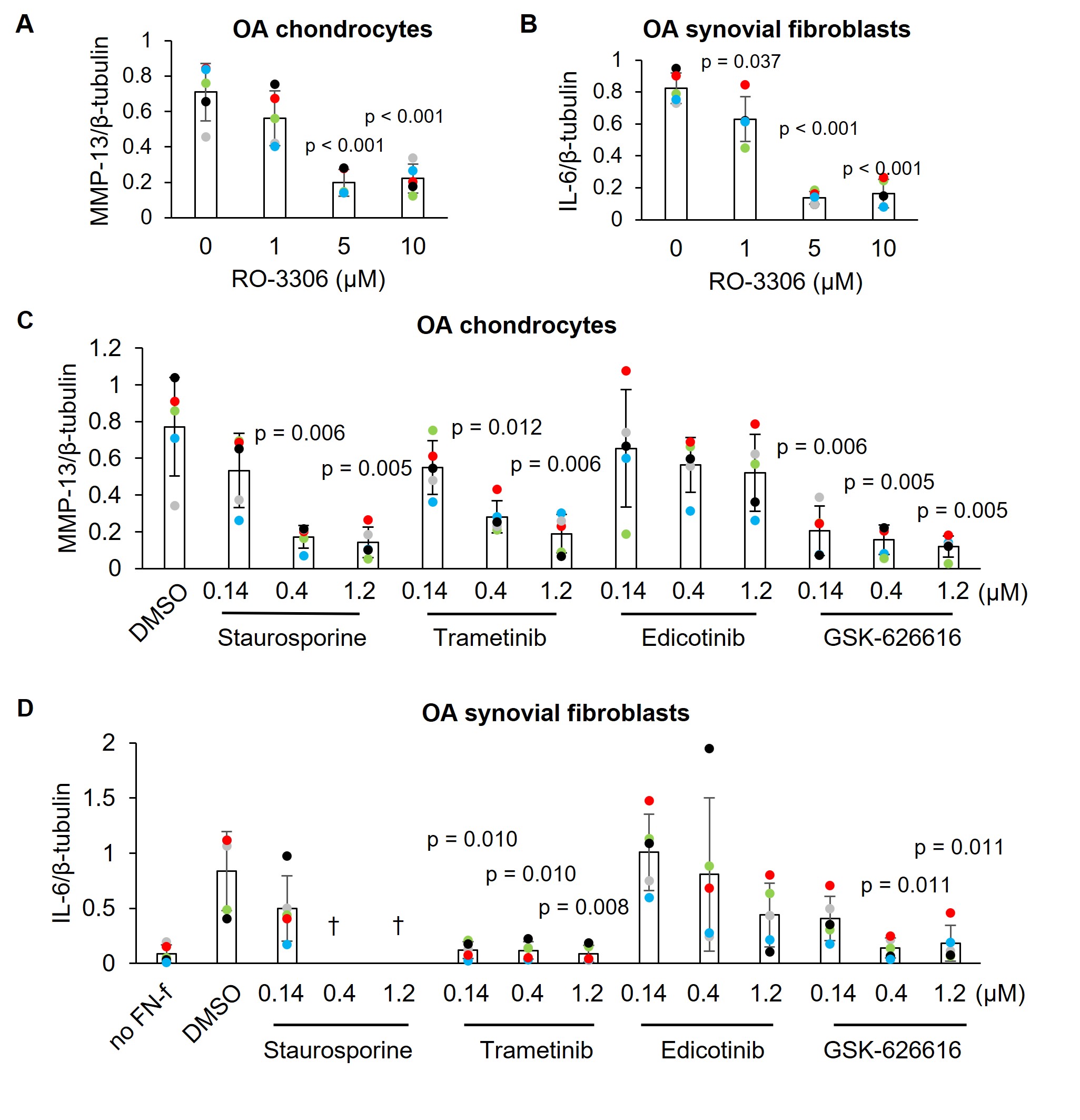
Results: Two pilot screens of 1344 small molecules (1280 from a commercially available LOPAC set at stamped at 10uM and 64 from a select kinase set in a dosing study) revealed 20 “hits” that inhibited FN7-10 induced MMP-13 by >80% without cytotoxicity (Tables 1 and 2). This included lorecivivint, used as a positive control. The screen identified several novel targets including CDK1 which was the target of two hits. In secondary testing in chondrocytes and synovial fibroblasts derived from OA patient joint tissues, CDK1, MEK1/2 and DYRK inhibitors blocked MMP-13 and IL-6 production by both cell types (Fig. 1). Staurosporine inhibited MMP-13 in chondrocytes but was cytotoxic in synovial fibroblasts while edicotinib inhibited MMP-13 and IL-6 but with considerable variability between donors.
Conclusion: We have successfully tested a novel HTS for OA drug discovery. Potential targets for OA therapeutic development were identified including CDK1 which inhibited MMP-13 and IL-6 production by OA chondrocytes and synovial fibroblasts. Given the lack of effective drugs on the market, the discovery of novel OA therapeutics that eliminate catabolic and inflammatory signaling in multiple joint tissue cell types would be a tremendous benefit to public health.
To cite this abstract in AMA style:
Coryell P, Pearce K, Hardy P, Chubinskaya S, Loeser R. A Novel Small Molecule Screening Assay Using Normal Human Chondrocytes Toward Osteoarthritis Drug Discovery [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/a-novel-small-molecule-screening-assay-using-normal-human-chondrocytes-toward-osteoarthritis-drug-discovery/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-novel-small-molecule-screening-assay-using-normal-human-chondrocytes-toward-osteoarthritis-drug-discovery/