Session Information
Date: Monday, November 13, 2023
Title: (0859–0885) Osteoarthritis & Joint Biology – Basic Science Poster
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Autoimmunity, trauma, or infection lead to devastating arthropathies, within enormous socioeconomic impact due to its high frequency and chronicity. The development of novel treatments remains a significant challenge. Moreover, disease a etiology, sequence and drug responsiveness are exceptionally patient-specific, underscoring the need for personalized therapeutic strategies. Invitro cultures and animal models have been helpful in identifying and describing the pathological processes in arthritis; still, they cannot predict individual responses to treatment due to the complexity of the synovium. In the present study we sought to establish a reproducible organoid model that emulates the complex biological interactions within the human synovium, mimicking pathological conditions of the inflamed joint.
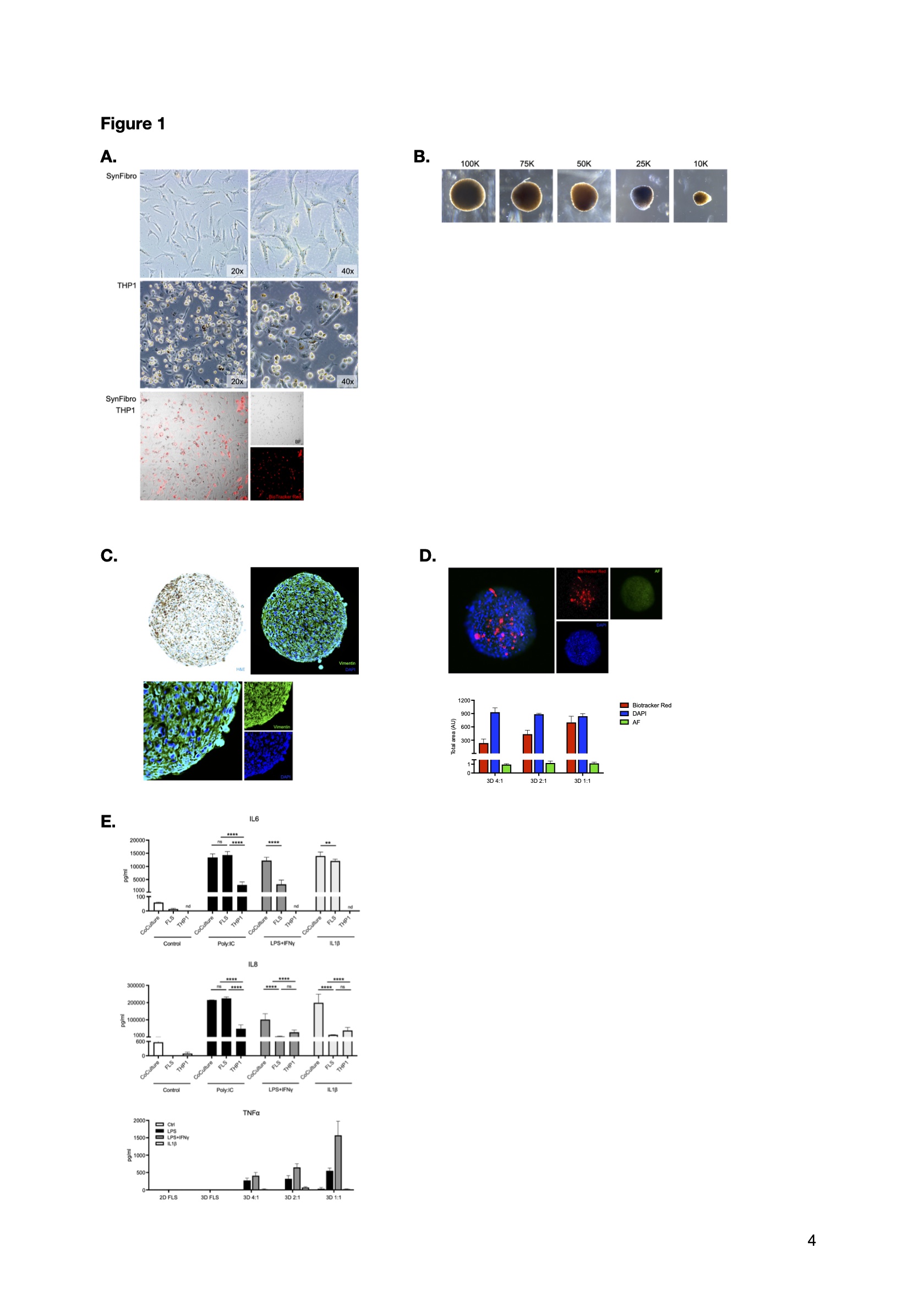
Methods: For the establishment of 2D and 3D cell cultures, native synovial fibroblasts and THP1 macrophages were utilized. Patient sample collection and biobank storage at the Rheumatology Department and Orthopedics Department USB was approved by the ethics review board EKNZ (2019-01391). Organoids were assembled by adding biocompatible magnetic nanoshuttles and subsequent bioprinting (m3D, Greiner BioOne), allowing self-assembly of cells into 3D structures by building autologous extracellular matrix. This highly scalable, reproducible, independent of artificial scaffolds approach, supports a native microenvironment preserving endogenous tissue phenotypes. Optimal setup parameters – growth status, cell number, size and culture conditions – organoid maturation, and medium formulation were tested, and inflammatory responses were assessed in the presence of RA-relevant stimuli by measuring the secretion of cytokines (ELISA) and the expression of cellular phenotypes (IHC).
Results: Biological material from 6 OA patients, including synovial tissue, plasma, serum, and synovial fluid was collected and stored. 2D and 3D synovial fibroblast and THP1 macrophage cultures (Figure 1A), cocultures (Figure 1B) and organoids (Figure 1C) were established. Macrophage incorporation in the synovial organoids was confirmed in different coculture schemes (Figure 1D). Data from a restricted set of RA-relevant markers displayed a synergistic effect of the coculture setting on proinflammatory cytokine secretion after stimulation; moreover, initial assessments comparing the 2D cultures and 3D organoids upon inflammatory stimulation showed that macrophages are the main source of TNFα (Figure 1E).
Conclusion: The establishment of an engineered human 3D synovial tissue model, emulating the human synovium by utilizing targeted tissue material, serves to functionally test essential aspects of synovial inflammation. The ability to induce inflammatory responses in the organoids provides a proof of concept for cytokine-driven inflammation in autoimmunity. This may serve as an important tool in drug development and personalized medicine, and tackle the limited predictive value of existing in vitro and in vivo animal models.
To cite this abstract in AMA style:
Tiaden A, Häner Massimi S, Walker U, Kyburz D, Giaglis S. A Novel 3D-Synovium-Immune Microenvironment Mimics Macrophage-Synovial Fibroblast Interactions in Inflammatory Arthropathies [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/a-novel-3d-synovium-immune-microenvironment-mimics-macrophage-synovial-fibroblast-interactions-in-inflammatory-arthropathies/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-novel-3d-synovium-immune-microenvironment-mimics-macrophage-synovial-fibroblast-interactions-in-inflammatory-arthropathies/