Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Both human and animal studies associate specific microbiota and microbial metabolic pathways with the development of RA and autoimmune arthritis, thereby providing a novel target for next-generation therapeutic development. Notably, carnitine and choline metabolism are associated with RA in humans and autoimmune arthritis in the collagen-induced arthritis (CIA) mouse model. Further, specific bacteria linked to disease in RA and CIA can metabolize dietary carnitine and choline to drive production of trimethylamine-N-oxide (TMAO). TMAO is implicated in the pathogenesis of inflammatory diseases, such as atherosclerosis, whose underlying disease processes mirror that of RA and CIA. Thus, we investigated modulation of TMAO production as a therapeutic target in CIA using the choline TMA lyase inhibitors 3,3-diemthyl-1-butanol (DMB) and fluoromethylcholine (FMC).
Methods: 6-week-old male DBA/1j mice were immunized at days 0 and 21 with bovine type II collagen (CII) in complete Freund’s adjuvant and either left untreated or treated with 1% (vol/vol) DMB, 1% (vol/vol) 3,3-dimethylbutyrate (DMBut), or 100mg/kg FMC. Mice were assessed for disease severity using established metrics until 35 days post-initial immunization. On day 35, mice were sacrificed and tissues were harvested for analysis. Multiplex serum cytokine immunoassays; serum anti-CII ELISAs; fecal 16S sequencing; serum and liver metabolomics; flow cytometry of splenocytes and inguinal lymph node lymphocytes; and cytokine ELISAs of bone marrow-derived macrophage (BMDM) supernatants were performed.
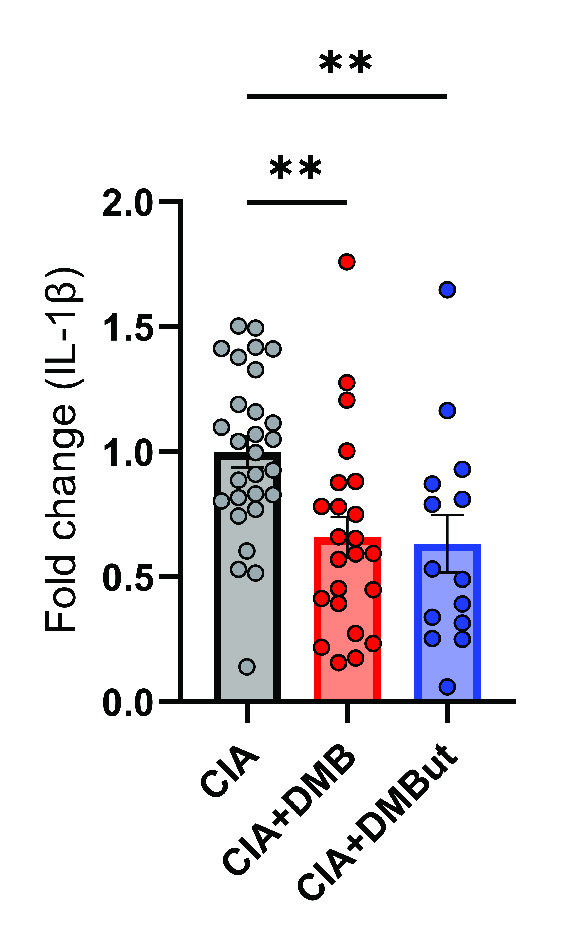
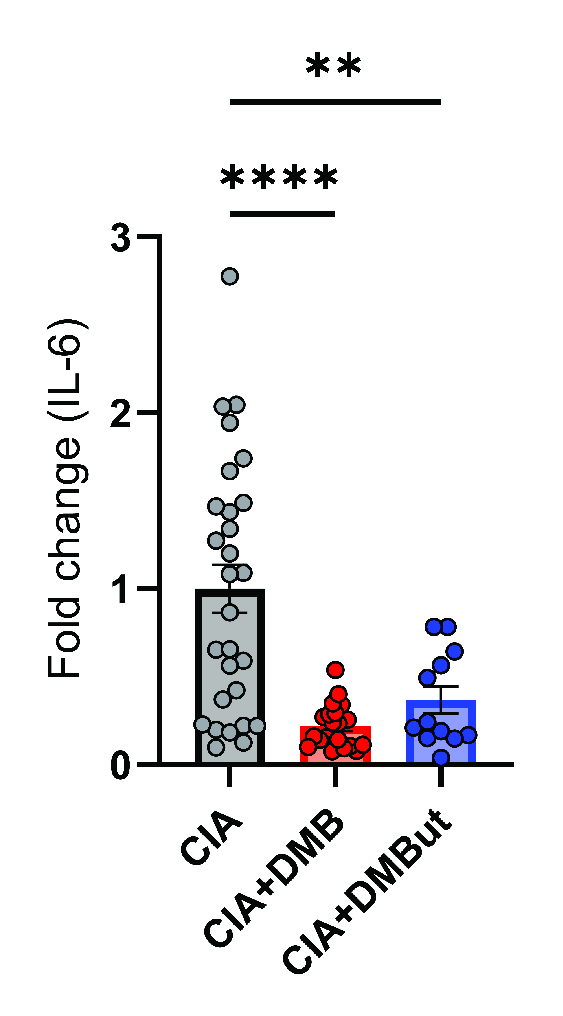
Results: Mice given DMB showed significant reduction ( >50%) in disease severity relative to untreated mice with CIA, while mice given FMC did not show reduced disease. FMC but not DMB significantly reduced cecal TMA and serum TMAO concentrations compared to untreated CIA mice. Fecal bacterial communities were not significantly altered by treatment with either FMC or DMB. DMB and its predicted metabolite, DMBut, were detected in the liver and serum, suggesting systemic effects, and both significantly reduced arthritis severity and circulating IL-1β and IL-6, but not circulating anti-CII antibodies, compared to untreated CIA mice. Splenic Th17 populations were significantly increased with DMBut treatment, but Th1, Tfh, and Treg populations were unchanged with DMB and DMBut treatment. Stimulation of BMDMs with LPS in the presence of DMB or DMBut significantly reduced secretion of IL-1β and IL-6.
Conclusion: We found that inhibition of microbial TMA/TMAO production using FMC does not reduce arthritis severity in CIA, while treatment with DMB ameliorates CIA seemingly independent of TMA/TMAO production or microbiome effects. Moreover, DMB and a product of its metabolism in the host, DMBut, both significantly reduce disease severity and pro-inflammatory cytokines in CIA and stimulated BMDMs, suggesting that DMB and/or its metabolites have anti-inflammatory effects on monocytes. Elucidating the mechanism by which these small molecules modulate disease may provide directions for developing future RA therapies.
To cite this abstract in AMA style:
Allen B, Fechtner S, Chriswell M, Jubair W, Vrolijk M, Holers V, Kuhn k. 3,3-dimethyl-1-butanol and Its Metabolite 3,3-dimethylbutyrate Ameliorate Arthritis Severity in CIA Independent of Choline TMA Lyase Activity [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/33-dimethyl-1-butanol-and-its-metabolite-33-dimethylbutyrate-ameliorate-arthritis-severity-in-cia-independent-of-choline-tma-lyase-activity/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/33-dimethyl-1-butanol-and-its-metabolite-33-dimethylbutyrate-ameliorate-arthritis-severity-in-cia-independent-of-choline-tma-lyase-activity/