Session Information
Session Type: Abstract Session
Session Time: 1:00PM-1:15PM
Background/Purpose: Previous reports show that malondialdehyde-acetaldehyde (MAA) adducts are overexpressed in RA, especially in joint/lung tissues, and that they colocalize with citrulline (CIT). This is relevant as MAA and CIT act synergistically through p38- and NF-κB-dependent pathways to activate macrophages, producing proinflammatory and profibrotic effects mediated through crosstalk with tissue resident cells. Heart failure (HF) is a highly overrepresented cause of death in RA patients, characterized by tissue inflammation, fibrosis, and vascular endothelial dysfunction. Herein, we sought to examine whether MAA has increased expression and colocalization with CIT in heart tissues from patients with RA-HF. We also examined whether inhibition of macrophage signaling pathways limits endothelial cell (EC) activation and inflammation mediated by MAA-CIT stimulation.
Methods: Left ventricular (LV) apex tissue from RA-HF patients and age/sex matched non-RA HF controls were examined (n=3/group). LV tissues were stained with anti-CIT and anti-MAA antibodies. Quantification and co-localization were done using ImageJ and FIJI Coloc2. Separately, human U-937 macrophages were pre-incubated with either media, 1 µM of BIRB-796 (p38 inhibitor), or 50 µM of BAY-11-7085 (NF-κB inhibitor) for 1-hr before stimulation with unmodified fibrinogen (FIB) or FIB co-modified with MAA+CIT (FIB-MAA-CIT) for 48-hrs. To assess the crosstalk between macrophages and ECs, macrophage supernatants were collected and used to indirectly stimulate human coronary artery endothelial cells (HCAECs) for 24-hrs. HCAEC supernatants were then analyzed for IL-6, MCP-1, ICAM-1, and VCAM-1 by ELISA, while RNA was analyzed by RT-PCR. Analyses were performed using a Student’s T test or one-way ANOVA with Tukey’s post hoc test.
Results: LV tissues from RA-HF patients demonstrated trends towards increased CIT deposition (p=0.09) and significantly increased MAA deposition (p< 0.05) vs. non-RA HF controls, with strong co-localization (R2=0.81) (Fig 1). HCAECs stimulated with supernatants from macrophages treated with FIB-MAA-CIT showed significantly increased expression of IL-6, MCP-1, ICAM-1, and VCAM-1 compared to FIB control, validating prior observations. Interestingly, direct stimulation of HCAECs with FIB-MAA-CIT yielded diminished effects (not shown). HCAECs stimulated with supernatants from macrophages pre-treated with p38 or NF-κB inhibitors and FIB-MAA-CIT showed significantly attenuated mRNA expression and soluble cytokine/chemokine production (Figs 2,3).
Conclusion: This study is the first to demonstrate increased MAA expression and MAA-CIT co-localization in cardiac tissues of RA-HF patients compared to non-RA HF patients. We also demonstrated that macrophages activated with FIB-MAA-CIT mediate coronary EC inflammation through their supernatants, an effect that is attenuated following inhibition of p38 and NF-κB. Further research will validate these findings using patient-derived monocytes and explore whether agents targeting MAA, CIT, or downstream signaling pathways have the potential to attenuate cardiac dysfunction that occurs in mouse models of RA.
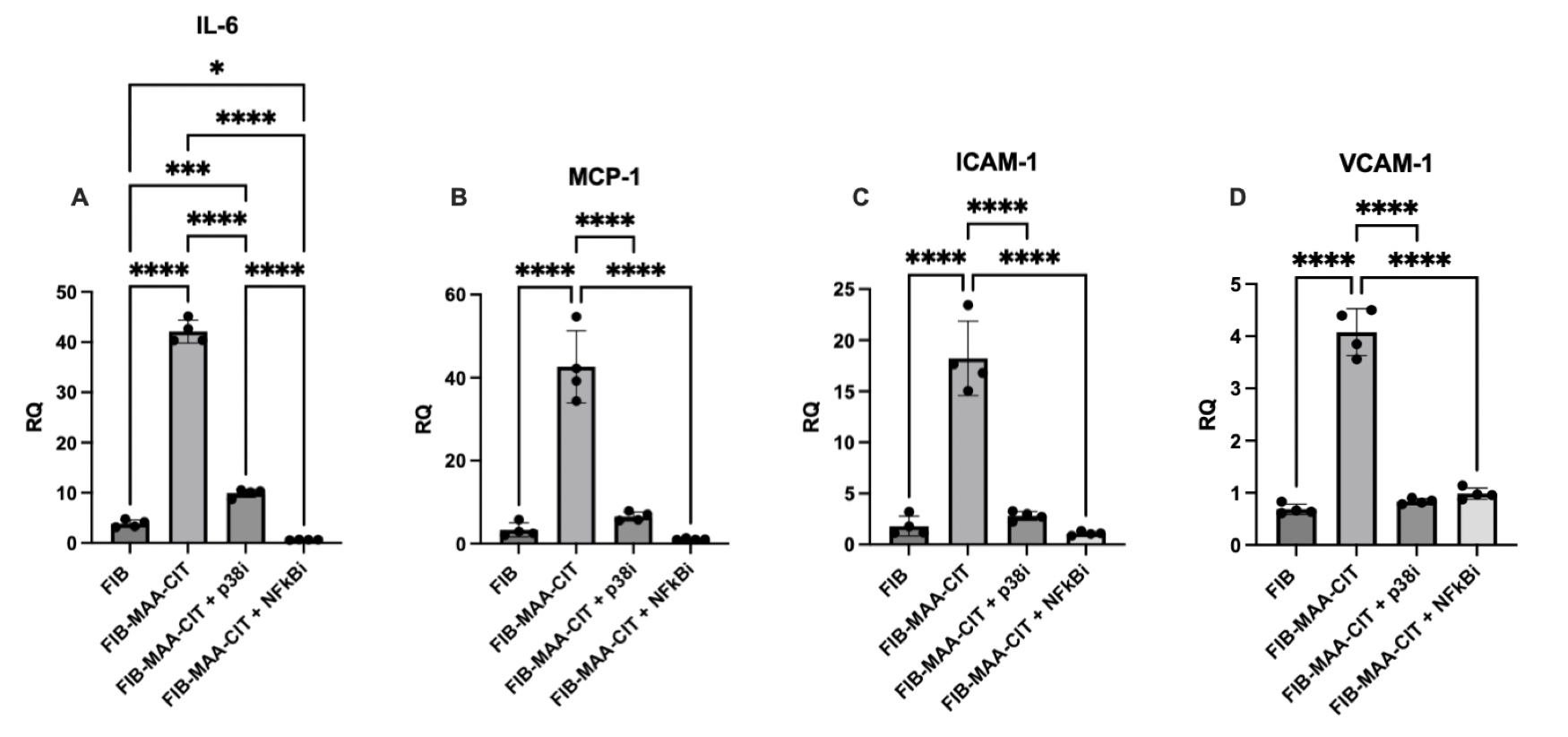 Figure 1. Immunohistochemistry Reveals CIT and MAA Expression and Colocalization in the Heart of RA-HF Patients. Representative image of 20X frame at apex of left ventricle is shown. White scale bar = 200 µm. Data shown on graphs are mean ± standard deviation. Student’s T test was performed and the statistical differences between groups are shown in *: * p < 0.05.
Figure 1. Immunohistochemistry Reveals CIT and MAA Expression and Colocalization in the Heart of RA-HF Patients. Representative image of 20X frame at apex of left ventricle is shown. White scale bar = 200 µm. Data shown on graphs are mean ± standard deviation. Student’s T test was performed and the statistical differences between groups are shown in *: * p < 0.05.
.jpg) Figure 2. RT-PCR Demonstrating Attenuated HCAEC Inflammatory Gene Expression Following Incubation with Supernatants from Macrophages Stimulated with FIB-MAA-CIT in the Presence or Absence of p38 and NF-κB Inhibitors. 24-hr mRNA expression was measured for; A) IL-6, B) MCP-1, C) ICAM-1, and D) VCAM-1. Relative quotient (RQ) compared to media-cultured negative control cells were calculated and shown in the y-axis. One-way ANOVA was performed and the statistical differences between groups are shown in *: * p < 0.05; *** p < 0.001; **** p < 0.0001.
Figure 2. RT-PCR Demonstrating Attenuated HCAEC Inflammatory Gene Expression Following Incubation with Supernatants from Macrophages Stimulated with FIB-MAA-CIT in the Presence or Absence of p38 and NF-κB Inhibitors. 24-hr mRNA expression was measured for; A) IL-6, B) MCP-1, C) ICAM-1, and D) VCAM-1. Relative quotient (RQ) compared to media-cultured negative control cells were calculated and shown in the y-axis. One-way ANOVA was performed and the statistical differences between groups are shown in *: * p < 0.05; *** p < 0.001; **** p < 0.0001.
.jpg) Figure 3. ELISA Demonstrating Attenuated HCAEC Inflammatory Protein Release Following Incubation with Supernatants from Macrophages Stimulated with FIB-MAA-CIT in the Presence or Absence of p38 and NF-κB Inhibitors. 24-hr cytokine concentrations in supernatants were measured for; A) IL-6, B) MCP-1, C) ICAM-1, and D) VCAM-1. One-way ANOVA was performed and the statistical differences between inhibitory groups are shown in *: ** p < 0.01; *** p < 0.001; **** p < 0.0001.
Figure 3. ELISA Demonstrating Attenuated HCAEC Inflammatory Protein Release Following Incubation with Supernatants from Macrophages Stimulated with FIB-MAA-CIT in the Presence or Absence of p38 and NF-κB Inhibitors. 24-hr cytokine concentrations in supernatants were measured for; A) IL-6, B) MCP-1, C) ICAM-1, and D) VCAM-1. One-way ANOVA was performed and the statistical differences between inhibitory groups are shown in *: ** p < 0.01; *** p < 0.001; **** p < 0.0001.
To cite this abstract in AMA style:
Johnson H, Zhou W, Duryee M, Sharp E, Sinanan K, Hunter C, Johnson T, Alfaidi M, Anderson D, Thiele G, Mikuls T. Malondialdehyde-Acetaldehyde and Citrulline Modified Proteins are Overexpressed in Cardiac Tissues in Rheumatoid Arthritis-Associated Heart Failure and Mediate Endothelial Cell Dysfunction [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/malondialdehyde-acetaldehyde-and-citrulline-modified-proteins-are-overexpressed-in-cardiac-tissues-in-rheumatoid-arthritis-associated-heart-failure-and-mediate-endothelial-cell-dysfunction/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/malondialdehyde-acetaldehyde-and-citrulline-modified-proteins-are-overexpressed-in-cardiac-tissues-in-rheumatoid-arthritis-associated-heart-failure-and-mediate-endothelial-cell-dysfunction/
