Session Information
Date: Monday, October 27, 2025
Title: (1553–1591) Systemic Sclerosis & Related Disorders – Clinical Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Gastrointestinal (GI) dysfunction is common and often debilitating in systemic sclerosis (SSc). Though it affects most patients, mechanisms are poorly understood and biomarkers for risk stratification and disease activity are limited. Here we sought to identify novel autoantibodies in patients with SSc GI disease and to determine whether they provide insight into clinical phenotype and disease mechanism.
Methods: Sera from patients from the Gastrointestinal Assessment Protocol (GAP), a subcohort of a large Scleroderma Research Registry, were screened for novel autoantibodies. Immunoprecipitations performed with murine myenteric plexus lysates were on-bead digested, and autoantigens were identified by mass spectrometry and then validated. Prevalence was determined, and clinical features associated with the validated autoantibodies were identified using both an all-comers cohort (n=179) and the GAP cohort (n=140). We also examined whether the identified specificities, anti-AGO and anti-DBT antibodies, associated with other known SSc antibodies using an unbiased clustering analysis. The expression of AGO2 and DBT in murine GI tract tissue was then examined by immunohistochemistry. Extracellular vesicles released by ENS and associated cells were captured from cultured longitudinal muscles – containing myenteric plexus (LM-MP) tissues, and their size, abundance, and presence of AGO protein as EV-associated cargo was ascertained by flow cytometry and western blot, respectively.
Results: Using sera from SSc patients with GI dysmotility, we identified AGO1, AGO2, and DBT as candidate autoantigens, which were validated in subsequent cohorts. Anti-AGO antibodies were present in 9.4% (17/179) and anti-DBT in < 1% (1/178) of patients in the all-comers cohort, but absent in healthy controls (0/38 for anti-AGO, 0/36 for anti-DBT). Anti-AGO–positive patients had significantly more GI-related social problems (p=0.02), stomachaches (p=0.005), bloating (p=0.02), and emotional distress including worry (p=0.012) and fear (p=0.02). In a GI-enriched validation cohort, anti-AGO antibodies were found in 11% (15/140), with higher titers correlating with more severe constipation (p=0.03) and bloating (p=0.02). Conversely, anti-DBT antibodies, present in 3% (4/126), were associated with significantly less constipation (p=0.02). Immunofluorescence demonstrated that anti-DBT antibodies specifically target mesodermal-derived enteric neurons and smooth muscle, while anti-AGO antibodies may target AGO2-containing extracellular vesicles (EV’s) released from the gut.
Conclusion: Antibodies in patients with SSc may provide insight into distinct clinical phenotypes and disease mechanism(s). Understanding the targets of the immune response and identifying immune biomarkers may shed light on important mechanisms of disease and novel therapies.
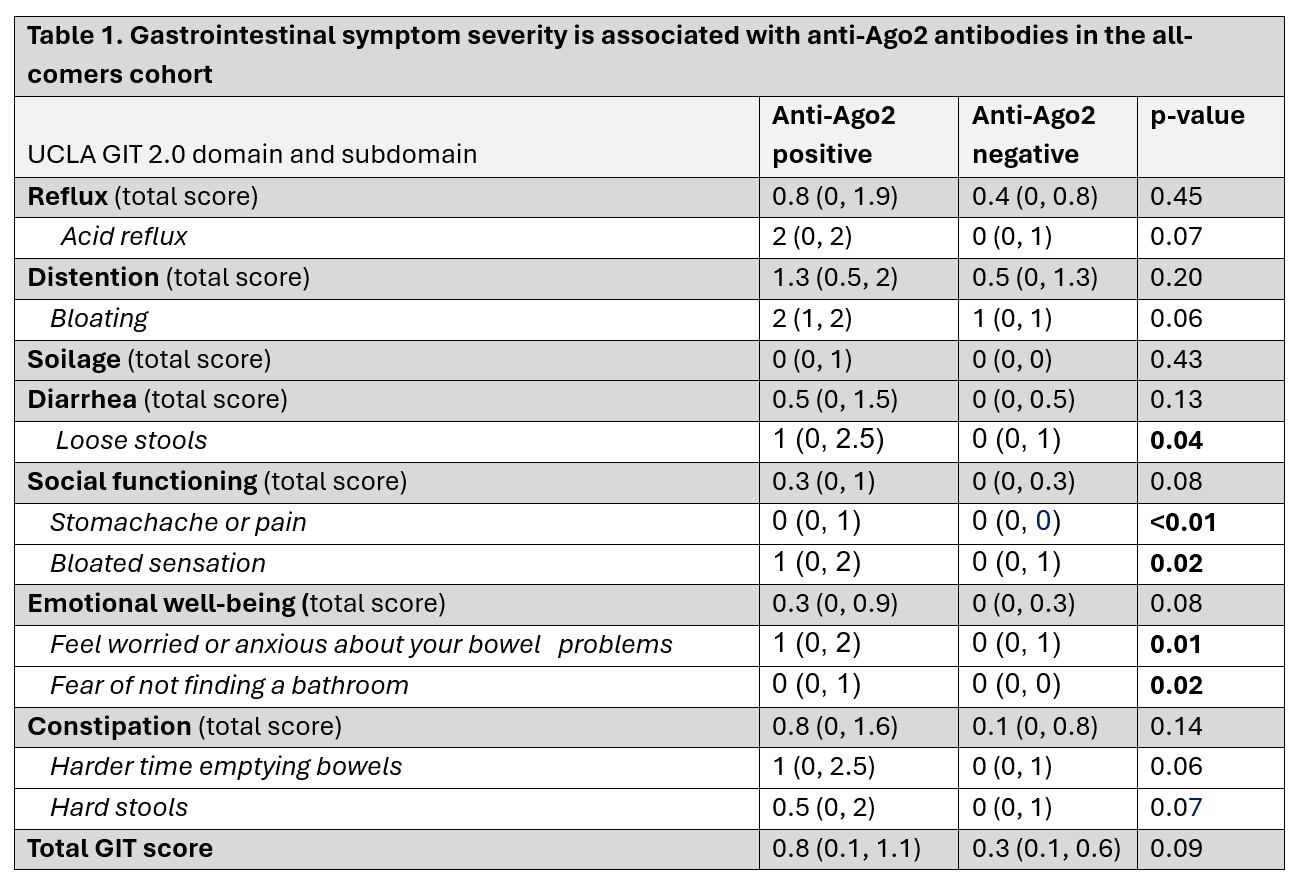 Figure 1: DBT staining of murine LMMP. Mitochondria-specific enzyme DBT is expressed abundantly by MENs in the adult ENS. Immunostaining adult murine (A) small intestinal and (B) colonic longitudinal muscle – containing myenteric plexus (LM-MP) tissues from a neural crest lineage fate mapping mouse model Wnt1-cre:tdTomato, where tdTomato (red) labels all derivatives of the neural crest, with commercially available anti-DBT antibodies (green), which labels the mitochondria-specific Dihydrolipoamide Branched Chain Transacylase (DBT) enzyme (also known as the E2 subunit of the branched-chain alpha-keto acid dehydrogenase complex (BCKD)) shows that DBT protein is localized specifically in the tdTomato-negative non-neural crest cells of the tissue. Mesoderm-derived enteric neurons (MENs, white arrows) in (A) small intestinal LM-MP show abundant expression of DBT, while (B) colonic MENs show relatively reduced abundance of MENs. Nuclei are labeled with DAPI (blue). Scale bar = 10 µm.
Figure 1: DBT staining of murine LMMP. Mitochondria-specific enzyme DBT is expressed abundantly by MENs in the adult ENS. Immunostaining adult murine (A) small intestinal and (B) colonic longitudinal muscle – containing myenteric plexus (LM-MP) tissues from a neural crest lineage fate mapping mouse model Wnt1-cre:tdTomato, where tdTomato (red) labels all derivatives of the neural crest, with commercially available anti-DBT antibodies (green), which labels the mitochondria-specific Dihydrolipoamide Branched Chain Transacylase (DBT) enzyme (also known as the E2 subunit of the branched-chain alpha-keto acid dehydrogenase complex (BCKD)) shows that DBT protein is localized specifically in the tdTomato-negative non-neural crest cells of the tissue. Mesoderm-derived enteric neurons (MENs, white arrows) in (A) small intestinal LM-MP show abundant expression of DBT, while (B) colonic MENs show relatively reduced abundance of MENs. Nuclei are labeled with DAPI (blue). Scale bar = 10 µm.
.jpg) Figure 2: AGO staining of LMMP. Small intestinal ENS releases AGO2-containing extracellular vesicles (EVs). (A) Immunostaining adult murine small intestinal longitudinal muscle – containing myenteric plexus (LM-MP) tissues with antibodies against neuronal marker Hu (green) and EV-marker AGO2 (Argonaut 2, red) shows presence of AGO2 around the Hu-labeled neurons, showing that the neurons release AGO2. (B) Short-term cultured preparations of adult murine small intestinal LM-MP release significant amounts of EVs in the culture media, as assessed by nanoparticle-detecting flow cytometry. (C) Western blot of isolated EVs from cultured LM-MP (shown in (B)) when assayed for the presence of AGO2 (estimated size of 99 kD) using anti-AGO2 antibodies shows a strong and specific band around 100kD providing evidence that small intestinal ENS releases abundant AGO2-containing EVs.
Figure 2: AGO staining of LMMP. Small intestinal ENS releases AGO2-containing extracellular vesicles (EVs). (A) Immunostaining adult murine small intestinal longitudinal muscle – containing myenteric plexus (LM-MP) tissues with antibodies against neuronal marker Hu (green) and EV-marker AGO2 (Argonaut 2, red) shows presence of AGO2 around the Hu-labeled neurons, showing that the neurons release AGO2. (B) Short-term cultured preparations of adult murine small intestinal LM-MP release significant amounts of EVs in the culture media, as assessed by nanoparticle-detecting flow cytometry. (C) Western blot of isolated EVs from cultured LM-MP (shown in (B)) when assayed for the presence of AGO2 (estimated size of 99 kD) using anti-AGO2 antibodies shows a strong and specific band around 100kD providing evidence that small intestinal ENS releases abundant AGO2-containing EVs.
To cite this abstract in AMA style:
McMahan Z, Pedroza C, Lee K, Chen B, Shah A, Hooper J, Puttapaka S, Casciola-Rosen L, Kulkarni S. Novel autoantibodies in patients with systemic sclerosis and gastrointestinal dysfunction provide insight into disease pathogenesis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/novel-autoantibodies-in-patients-with-systemic-sclerosis-and-gastrointestinal-dysfunction-provide-insight-into-disease-pathogenesis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/novel-autoantibodies-in-patients-with-systemic-sclerosis-and-gastrointestinal-dysfunction-provide-insight-into-disease-pathogenesis/

.jpg)