Session Information
Date: Monday, October 27, 2025
Title: (1123–1146) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster I
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: While aggregated neutrophil extracellular traps (aggNETs) constitute the primary structural component of tophi, the susceptible population for tophaceous gout remains poorly characterized. We investigated a familial case of early-onset tophaceous gout to elucidate novel mechanisms underlying tophus formation.While aggregated neutrophil extracellular traps (aggNETs) constitute the primary structural component of tophi, the susceptible population for tophaceous gout remains poorly characterized. We investigated a familial case of early-onset tophaceous gout to elucidate novel mechanisms underlying tophus formation.
Methods: Whole-exome sequencing was performed on a multigenerational family with early-onset tophaceous gout affecting the proband, his father, and brother. Molecular cloning generated wild-type and mutant macrophage migration inhibitory factor (MIF) expression vectors, with subsequent structural and functional characterization using three-dimensional modeling and circular dichroism spectroscopy. Tophaceous mice administered by MIF or mutant MIF vectors, Mif knockout mice and in vitro models assessed tophus degradation dynamics. Transwell assays with fluorescence labeling evaluated macrophage-mediated phagocytosis and intracellular processing of aggNETs.
Results: We identified a pathogenic MIF variant (p.Ile65Met, hereafter referred to as I65M) segregating with disease in the affected family. Structural analysis revealed this mutation induces conformational changes in the PD-D/E(X)K domain. Functional studies demonstrated: (1) significantly reduced nuclease activity of MIF(I65M) compared to wild-type; (2) impaired tophus degradation capacity of MIF(I65M) in both murine models and in vitro systems; (3) preserved macrophage phagocytosis but defective cytoplasmic degradation of aggNETs through a lysosome-independent pathway.
Conclusion: This study establishes a novel pathogenesis framework for familial tophaceous gout, demonstrating how MIF mutations lead to aggNETs accumulation through impaired clearance mechanisms. Our findings identify defective aggNETs degradation as a central pathophysiological process, offering new therapeutic targets for tophaceous gout management.
 Patient with tophi, WES, and pedigree: (A) The proband P1 (index case) in this study presented with tophi covering both hands. (B) Whole-exome sequencing revealed that the proband P1, his brother P2, and father P3 (not shown) all shared a heterozygous missense mutation in MIF (NM_002415.2):c.195C>G (p.Ile65Met) in exon 2. Additionally, proband P1 carried a homozygous missense mutation in MTHFR (NM_005957.5):c.665C>T (p.Ala222Val) in exon 5 and a heterozygous frameshift mutation in UBAPI (NM_001171201.1):c.192dup (p.Arg65SerfsTer18) in exon 1. (C) The pedigree chart showed that the proband’s father (P3) and brother (P2) were also early-onset tophaceous gout patients, with all three (P1, P2, P3) carrying the heterozygous MIF mutation, while P1 has two asymptomatic sons (aged 6 and 4). (D) In vitro pulsed-field gel electrophoresis using human genomic DNA as the substrate was performed to assess the nuclease activity of DNase-I and MIF. (E) The results demonstrate that wild-type MIF exhibits DNA-degrading activity, whereas the nuclease activity of the MIF (I65M) mutant is significantly reduced compared to the wild type.
Patient with tophi, WES, and pedigree: (A) The proband P1 (index case) in this study presented with tophi covering both hands. (B) Whole-exome sequencing revealed that the proband P1, his brother P2, and father P3 (not shown) all shared a heterozygous missense mutation in MIF (NM_002415.2):c.195C>G (p.Ile65Met) in exon 2. Additionally, proband P1 carried a homozygous missense mutation in MTHFR (NM_005957.5):c.665C>T (p.Ala222Val) in exon 5 and a heterozygous frameshift mutation in UBAPI (NM_001171201.1):c.192dup (p.Arg65SerfsTer18) in exon 1. (C) The pedigree chart showed that the proband’s father (P3) and brother (P2) were also early-onset tophaceous gout patients, with all three (P1, P2, P3) carrying the heterozygous MIF mutation, while P1 has two asymptomatic sons (aged 6 and 4). (D) In vitro pulsed-field gel electrophoresis using human genomic DNA as the substrate was performed to assess the nuclease activity of DNase-I and MIF. (E) The results demonstrate that wild-type MIF exhibits DNA-degrading activity, whereas the nuclease activity of the MIF (I65M) mutant is significantly reduced compared to the wild type.
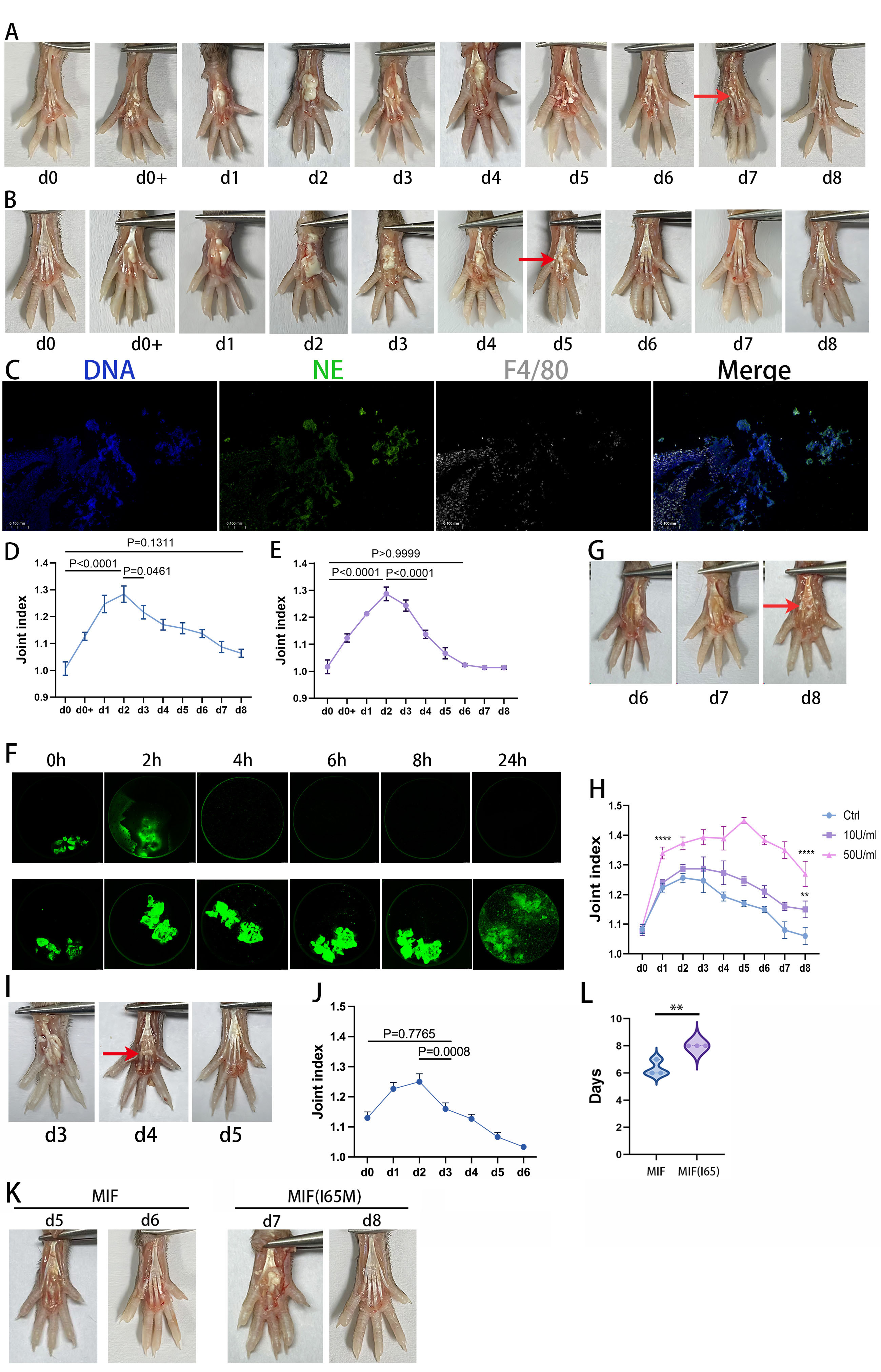
.jpg) DNA Enzyme Intervention Promotes Tophus Resolution: (A) Classic MSU crystal-induced gouty arthritis model in mouse footpads: One footpad was injected with MSU crystals (40 µL, 25 mg/mL), while the contralateral side received an equal volume of PBS. Tophus-like material (red arrow) appeared at 8 h post-injection (d0+) and spontaneously resolved by day 8 (d8). (B) Daily footpad injection of DNase-I (20 µL, 1600 U/mL) after MSU crystal injection accelerated tophus resolution, with complete disappearance by day 6 (d6, red arrow). (C) Immunofluorescence staining of d2 tophus-like material revealed abundant NETs containing macrophages. (D) Joint swelling index in the MSU crystal-injected group returned to baseline (no statistical difference vs. d0) by day 8. (E) The DNase-I + MSU crystal group showed recovery of joint swelling index by day 6. (F) In vitro, aggNETs (formed by co-culturing neutrophils [1.2×10⁷/mL] with MSU crystals [0.6 mg/10⁶ cells]) were stained with Sytox (DNA). DNase-I treatment reduced aggNETs within 2 h, with near-complete dissolution at 4 h. (G, H) Uricase (10 U/mL, 50 U/mL) co-injection with MSU crystals failed to resolve tophi by day 8, with significantly higher arthritis indices vs. controls. (I, J) Combined uricase (50 U/mL) + DNase-I daily intervention achieved tophus resolution by day 5 and restored joint swelling index by day 3. (K, L) In MSU crystal-induced arthritis, MIF-treated mice showed tophus resolution at day 6.3, while MIF (I65M)-treated mice required 8 days (significantly delayed, **P < 0.01, ****P < 0.0001).
DNA Enzyme Intervention Promotes Tophus Resolution: (A) Classic MSU crystal-induced gouty arthritis model in mouse footpads: One footpad was injected with MSU crystals (40 µL, 25 mg/mL), while the contralateral side received an equal volume of PBS. Tophus-like material (red arrow) appeared at 8 h post-injection (d0+) and spontaneously resolved by day 8 (d8). (B) Daily footpad injection of DNase-I (20 µL, 1600 U/mL) after MSU crystal injection accelerated tophus resolution, with complete disappearance by day 6 (d6, red arrow). (C) Immunofluorescence staining of d2 tophus-like material revealed abundant NETs containing macrophages. (D) Joint swelling index in the MSU crystal-injected group returned to baseline (no statistical difference vs. d0) by day 8. (E) The DNase-I + MSU crystal group showed recovery of joint swelling index by day 6. (F) In vitro, aggNETs (formed by co-culturing neutrophils [1.2×10⁷/mL] with MSU crystals [0.6 mg/10⁶ cells]) were stained with Sytox (DNA). DNase-I treatment reduced aggNETs within 2 h, with near-complete dissolution at 4 h. (G, H) Uricase (10 U/mL, 50 U/mL) co-injection with MSU crystals failed to resolve tophi by day 8, with significantly higher arthritis indices vs. controls. (I, J) Combined uricase (50 U/mL) + DNase-I daily intervention achieved tophus resolution by day 5 and restored joint swelling index by day 3. (K, L) In MSU crystal-induced arthritis, MIF-treated mice showed tophus resolution at day 6.3, while MIF (I65M)-treated mice required 8 days (significantly delayed, **P < 0.01, ****P < 0.0001).
.jpg) MIF Promotes Macrophage Phagocytosis of aggNETs and the Spatial Conformation of MIF (I65M): (A, C) THP-1-derived macrophages phagocytosing aggNETs. DNA (aggNETs) was stained with Sytox Green (intracellular live-cell DNA remains unstained). Over time, fluorescent signal appeared within macrophages, with gradually decreasing mean fluorescence intensity (MFI). (B) MIF-knockdown THP-1-derived macrophages exhibited significantly impaired phagocytosis of aggNETs. (D) MIF enhanced THP-1 phagocytosis of aggNETs, while MIF (I65M) showed reduced activity (peak phagocytosis at 4 h; MIF group displayed strongest intracellular green fluorescence, markedly weaker in MIF (I65M) group). (E) Schematic of human MIF’s three PD-D/E(X)K domains (blue, green, purple) with amino acid sequences. Red “I→M” indicates the mutation site. (F) 3D structure of human MIF showing the mutation site (red) located in the 4th β-sheet between PD-E (blue) and PD-D (purple).
MIF Promotes Macrophage Phagocytosis of aggNETs and the Spatial Conformation of MIF (I65M): (A, C) THP-1-derived macrophages phagocytosing aggNETs. DNA (aggNETs) was stained with Sytox Green (intracellular live-cell DNA remains unstained). Over time, fluorescent signal appeared within macrophages, with gradually decreasing mean fluorescence intensity (MFI). (B) MIF-knockdown THP-1-derived macrophages exhibited significantly impaired phagocytosis of aggNETs. (D) MIF enhanced THP-1 phagocytosis of aggNETs, while MIF (I65M) showed reduced activity (peak phagocytosis at 4 h; MIF group displayed strongest intracellular green fluorescence, markedly weaker in MIF (I65M) group). (E) Schematic of human MIF’s three PD-D/E(X)K domains (blue, green, purple) with amino acid sequences. Red “I→M” indicates the mutation site. (F) 3D structure of human MIF showing the mutation site (red) located in the 4th β-sheet between PD-E (blue) and PD-D (purple).
To cite this abstract in AMA style:
wang Y, Zhu L, Han J, Qian J, Herrmann M, Xue J, Liu L. Variant Drives Tophus Formation through Dual Mechanisms: Extracellular Aggregation andvImpaired Macrophage Phagocytic Clearance [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/variant-drives-tophus-formation-through-dual-mechanisms-extracellular-aggregation-andvimpaired-macrophage-phagocytic-clearance/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/variant-drives-tophus-formation-through-dual-mechanisms-extracellular-aggregation-andvimpaired-macrophage-phagocytic-clearance/
