Session Information
Date: Monday, October 27, 2025
Title: Abstracts: Systemic Sclerosis & Related Disorders – Clinical II (0879–0884)
Session Type: Abstract Session
Session Time: 10:15AM-10:30AM
Background/Purpose: Interstitial lung disease (ILD) is a leading cause of death in patients with systemic sclerosis (SSc), especially in case of extensive disease detected on computed tomography (CT). Automated post-processing analysis software can compute the extent of ILD through lung texture analysis (LTA) of CT images. However, its use in clinical practice is limited by the lack of standardized thresholds.The aim of our study was to identify and validate optimal thresholds of ILD extent quantified through LTA to detect the presence of ILD and to define extensive ILD in SSc patients.
Methods: SSc patients visiting two SSc referral center between 2005-2021, who underwent chest CT, were included. Technically suitable images (non-contrast, axial inspiratory acquisition, slice thickness and spacing ≤2 mm) were analyzed through LTA (Imbio), quantifying the percentage of lung volume occupied by total ILD (sum of ground glass, reticulation and honeycombing). Two radiologists blinded to the LTA data independently reviewed the CT scans to visually identify the presence of ILD and extensive ILD. The latter was defined as ILD involvement >20% of lung parenchyma or forced vital capacity < 70% predicted in case of indeterminate extent (Goh 2008). Disagreements were resolved by consensus, otherwise through a third reviewer. Patients were randomly split into derivation and validation cohorts using a 2:1 ratio. Receiver operating characteristic (ROC) curves with area under the curve (AUC) were computed to identify the optimal ILD extent threshold for detecting presence of ILD and, in patients with ILD, for identifying extensive disease, using the visual evaluation as the reference standard. Kaplan Meier curves and Cox regression analysis were performed to determine the impact of ILD and extensive disease on both visual and LTA assessments on mortality up to 5 years, the second adjusted for known mortality risk factors (age, sex, diffuse skin subset and pulmonary hypertension).
Results: A total of 664/1118 (58%) SSc patients were eligible for the study (Table 1). Visual analysis identified ILD in 313 (47%) cases, 103 (33%) showing extensive ILD. In the derivation cohort (433 patients, 206 ILD, 38% extensive), ROC analysis identified the optimal total ILD extent threshold for ILD detection at 1% (AUC 0.83) and at 7% for extensive ILD (AUC 0.84). In the validation cohort (231 subjects, 104 ILD, 25% extensive), these thresholds achieved 78% sensitivity / 71% specificity for ILD detection, and 81% sensitivity / 70% specificity for extensive ILD identification. Over median 5 years follow-up, 84 (13%) patients died [37 (12%) in the ILD group]. After adjustment for confounders, both ILD presence (Fig. 1) and extensive disease (Fig. 2) were independent risk factors for mortality, with comparable results by visual and LTA assessments.
Conclusion: Cutoffs for total ILD extent by LTA to detect ILD presence and identify extensive disease were derived and validated, with comparable performance to visual evaluation on negative prognostic impact. Our results lay the foundation for expanding the use of LTA post-processing in SSc-ILD towards automated diagnosis and prognostic stratification.
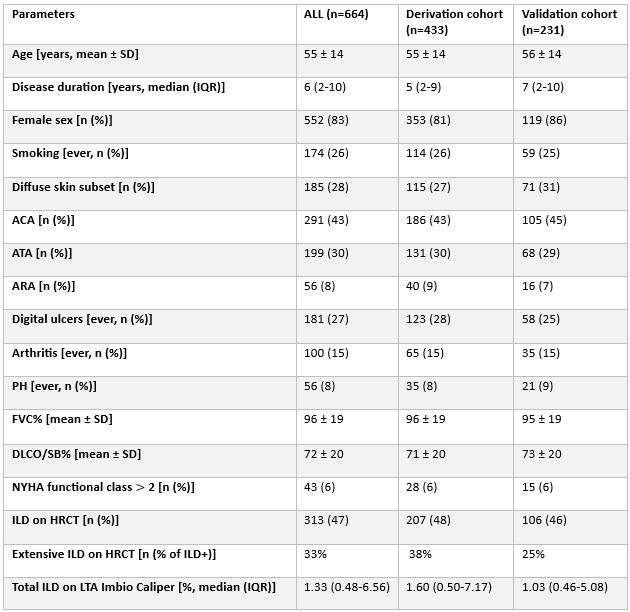 Table 1. Description of the study population, stratified into derivation and validation cohorts.
Table 1. Description of the study population, stratified into derivation and validation cohorts.
ACA: anticentromere antibodies; ARA: anti-RNA polymerase 3 antibodies; ATA: anti-topoisomerase I antibodies; ATA: DLCO: diffusion capacity of the lung for carbon oxide; FVC: forced vital capacity; ILD: interstitial lung disease; IQR: interquartile range; PH: pulmonary hypertension; SD: standard deviation.
.jpg) Figure 1. Prediction of mortality for interstitial lung disease identified through visual assessment vs. lung texture analysis.
Figure 1. Prediction of mortality for interstitial lung disease identified through visual assessment vs. lung texture analysis.
ILD: interstitial lung disease; HR: hazard ratio; LTA: lung texture analysis.
.jpg) Figure 2. Prediction of mortality for extensive (EXT) versus limited (LIM) interstitial lung disease (ILD) identified through visual assessment vs. lung texture analysis (LTA).
Figure 2. Prediction of mortality for extensive (EXT) versus limited (LIM) interstitial lung disease (ILD) identified through visual assessment vs. lung texture analysis (LTA).
EXT: extensive disease; ILD: interstitial lung disease; HR: hazard ratio; LIM: limited disease; LTA: lung texture analysis; PH: pulmonary hypertension.
To cite this abstract in AMA style:
landini N, Jungblut l, strappa c, Blüthgen C, Dobrota R, Elhai M, Mihai C, Muraru-Carbune S, Orlandi M, Occhipinti m, El-Aoufy K, Lepri G, panebianco v, larici a, Hoffmann-Vold A, Nardi C, Guiducci S, Bellando-Randone S, Matucci-Cerinic M, Frauenfelder T, Distler O, Bruni C. Detecting Interstitial Lung Disease and Identifying Extensive Disease on Chest Computed Tomography in Patients with Systemic Sclerosis: Cut-Offs for Lung Texture Analysis and its Prognostic Implications [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/detecting-interstitial-lung-disease-and-identifying-extensive-disease-on-chest-computed-tomography-in-patients-with-systemic-sclerosis-cut-offs-for-lung-texture-analysis-and-its-prognostic-implicatio/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/detecting-interstitial-lung-disease-and-identifying-extensive-disease-on-chest-computed-tomography-in-patients-with-systemic-sclerosis-cut-offs-for-lung-texture-analysis-and-its-prognostic-implicatio/
