Session Information
Date: Sunday, October 26, 2025
Title: Abstracts: Rheumatoid Arthritis – Etiology and Pathogenesis (0795–0800)
Session Type: Abstract Session
Session Time: 1:45PM-2:00PM
Background/Purpose: Fibroblasts are critical in promoting pathogenic synovial inflammation and bone erosion in rheumatoid arthritis (RA). Moreover, recent studies have pointed to their potential contribution to treatment-refractory disease, highlighting the value of targeting these cells therapeutically. Our prior work identified Wnt signaling as a novel mechanism driving stromal inflammation. In response to non-canonical Wnt activation, human synovial fibroblasts markedly increase production of inflammatory cytokines and chemokines in vitro. Further, we found that Wnt activation increases inflammatory arthritis severity and duration in vivo in a mouse model. Our analyses of human disease show that fibroblast Wnt activation signatures are increased in RA synovium and across other inflammatory conditions. Here, we investigate the therapeutic potential of Wnt pathway blockade and demonstrate that inhibiting Wnt signaling reduces arthritis severity and synovial inflammation in a mouse model of inflammatory arthritis.
Methods: C57Bl/6 mice underwent antigen-induced arthritis (AIA) by subcutaneous administration of methylated BSA (mBSA) at day -21 followed by intraarticular knee injection of mBSA at day 0. Mice were given daily intraperitoneal injections of the Wnt signaling inhibitor LGK974 or a vehicle control on days 0-4. Knee thickness and clinical scores were assessed. Synovial tissue was collected at day 4 and processed for H&E, flow cytometry analysis, cell sorting and single-cell RNA sequencing.
Results: To investigate whether the abrogation of Wnt signaling would reduce the severity of inflammatory arthritis, we utilized the AIA model and treated mice with LGK974, a pharmacologic inhibitor of Wnt ligand secretion. Mice treated with LGK974 exhibited a marked decrease in knee swelling (Fig 1A) and clinical arthritis scores (Fig 1B) compared to vehicle controls. Histologic analyses of knee joints collected at day 4 revealed decreased lining hyperplasia and leukocyte infiltration (Fig 1C-D). Moreover, flow cytometry analyses of cells isolated from synovial tissue showed decreased absolute numbers of leukocytes and a trend towards reduced neutrophil counts (Fig 1E). While total numbers of fibroblasts did not significantly differ in synovial tissue from LGK974- and control-treated mice (Fig 2A), both lining and sublining fibroblasts (Fig 2B) exhibited changes in transcriptomic phenotype. Specifically, synovial fibroblasts from LGK974-treated mice showed decreased expression of CXCL5, a chemokine that participates in neutrophil recruitment in RA and that is increased upon Wnt administration (Fig 2C). They also upregulated SOCS1 and SOCS3, inhibitors of JAK signaling (Fig 2D). These results suggest that blocking Wnt signaling dampens synovial fibroblast inflammatory activation during arthritis and, in turn, their ability to recruit and activate infiltrating leukocytes.
Conclusion: Collectively, our findings demonstrate the utility of Wnt pathway inhibition in reducing inflammatory arthritis severity. This work identifies a novel fibroblast-targeted therapeutic avenue for RA with a compelling potential value for patients whose disease is recalcitrant to conventional treatment modalities.
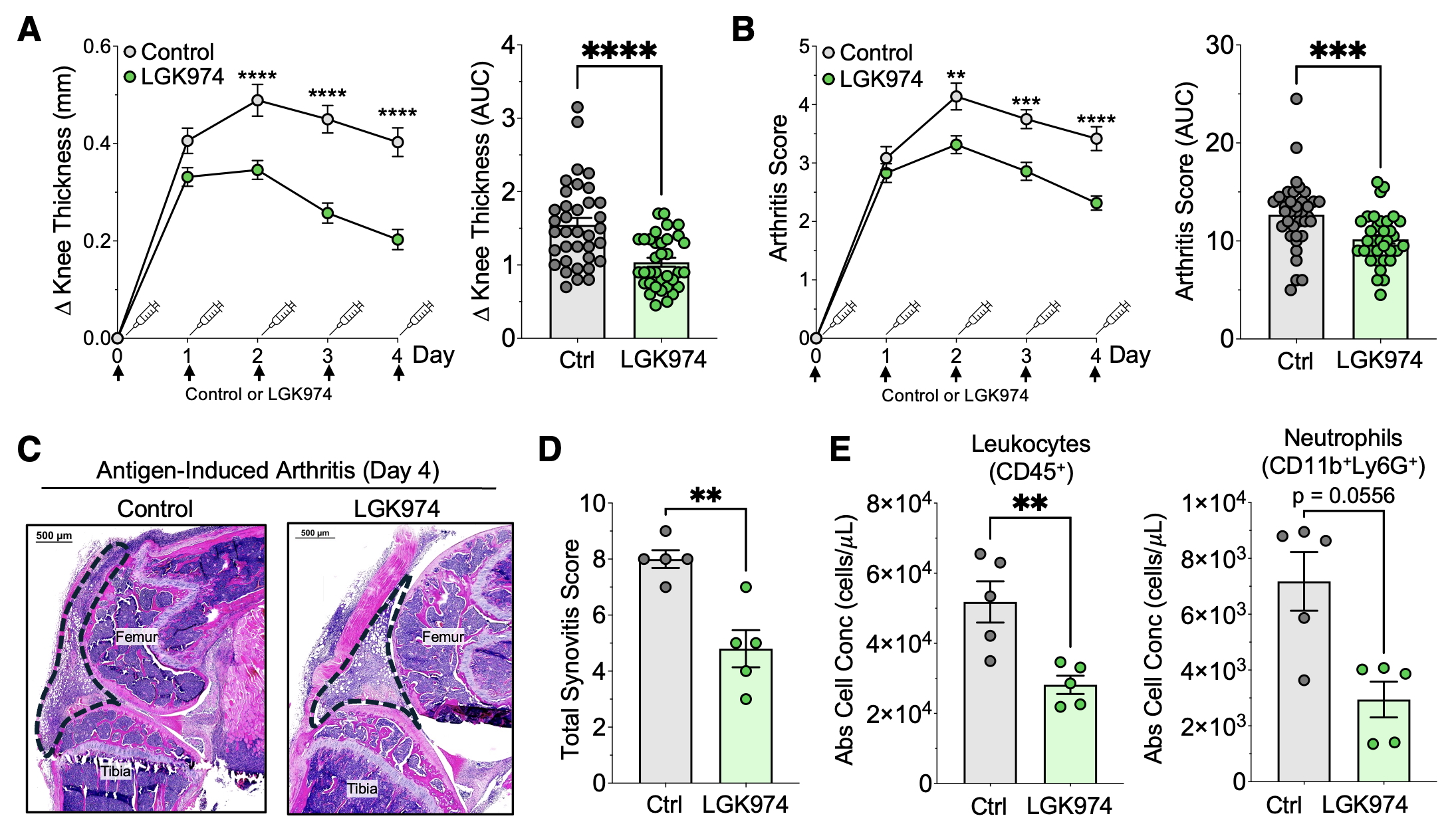 Figure 1: Inhibition of Wnt signaling reduces arthritis severity and leukocyte infiltration in a mouse model of inflammatory arthritis. (A-B) Plots and AUC calculations for knee thickness measurements (A) and clinical arthritis scores (B) at the time points indicated from mice that underwent antigen-induced arthritis (AIA) and have been treated with the Wnt signaling inhibitor LGK974 (n=35) or a vehicle control (n=36). (C-D) Representative H&E images (C) and histology synovitis scores (D) from knee joints taken from AIA mice treated with LGK975 (n=5) or control (n=5). Dotted lines indicate outline of synovial infiltrate. (E) Flow cytometry data showing absolute numbers of leukocytes and neutrophils derived from knee synovial tissue of AIA mice treated with LGK974 (n=5) or control (n=5).
Figure 1: Inhibition of Wnt signaling reduces arthritis severity and leukocyte infiltration in a mouse model of inflammatory arthritis. (A-B) Plots and AUC calculations for knee thickness measurements (A) and clinical arthritis scores (B) at the time points indicated from mice that underwent antigen-induced arthritis (AIA) and have been treated with the Wnt signaling inhibitor LGK974 (n=35) or a vehicle control (n=36). (C-D) Representative H&E images (C) and histology synovitis scores (D) from knee joints taken from AIA mice treated with LGK975 (n=5) or control (n=5). Dotted lines indicate outline of synovial infiltrate. (E) Flow cytometry data showing absolute numbers of leukocytes and neutrophils derived from knee synovial tissue of AIA mice treated with LGK974 (n=5) or control (n=5).
.jpg) Figure 2: Wnt pathway blockade decreases the inflammatory potentiation of synovial fibroblasts during arthritis. (A) Flow cytometry showing absolute numbers of fibroblast populations in AIA mice treated with LGK974 (n=5) or vehicle control (n=5). (B) UMAP showing 34,728 fibroblasts from AIA mice treated with LGK974 (n=3) or vehicle control (n=3). PRG4+ lining, THY1+ sublining, and MKI67+ proliferative fibroblast subpopulations are depicted. (C-D) Violin plots showing expression of the chemokine CXCL5 (C) and immune regulators SOCS1 and SOCS3 (D) in sublining and lining fibroblasts from single-cell RNA sequencing of synovial tissue from AIA mice treated with LGK974 (n=3) or control (n=3).
Figure 2: Wnt pathway blockade decreases the inflammatory potentiation of synovial fibroblasts during arthritis. (A) Flow cytometry showing absolute numbers of fibroblast populations in AIA mice treated with LGK974 (n=5) or vehicle control (n=5). (B) UMAP showing 34,728 fibroblasts from AIA mice treated with LGK974 (n=3) or vehicle control (n=3). PRG4+ lining, THY1+ sublining, and MKI67+ proliferative fibroblast subpopulations are depicted. (C-D) Violin plots showing expression of the chemokine CXCL5 (C) and immune regulators SOCS1 and SOCS3 (D) in sublining and lining fibroblasts from single-cell RNA sequencing of synovial tissue from AIA mice treated with LGK974 (n=3) or control (n=3).
To cite this abstract in AMA style:
Mueller A, Zou A, Marsh L, Kemble S, Nayar S, Watts G, Murphy C, Taylor E, Major T, Gardner D, Buckley C, Wei K, Raychaudhuri S, Korsunsky i, Filer A, Croft A, Brenner M. Inhibition of Wnt Signaling Attenuates Inflammatory Arthritis Severity [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/inhibition-of-wnt-signaling-attenuates-inflammatory-arthritis-severity/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/inhibition-of-wnt-signaling-attenuates-inflammatory-arthritis-severity/
