Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: T cell receptor (TCR) signaling strength is a key determinant of immune tolerance and autoimmunity, yet the threshold needed to prevent pathogenic self-reactivity remains poorly defined. ZAP70 is a critical kinase in the TCR signaling cascade, and the SKG and YYAA mice carry distinct hypomorphic ZAP70 mutations—resulting in different degrees of impaired TCR signaling, more severe in SKG. Both strains generate rheumatoid factor (RF) autoantibodies, but only SKG mice develop CD4⁺ T cell mediated erosive arthritis. This divergence allows us to define how TCR signaling thresholds shape CD4⁺ T cell fate and identify the minimum signaling needed to maintain tolerance and prevent arthritis.
Methods: SKG and YYAA mice were crossed to Nur77-eGFP reporter mice to quantify TCR signal strength during thymic development and in the periphery. We analyzed expression of Nur77-eGFP, inhibitory receptors, and TCR Vβ repertoire by flow cytometry in thymocyte subsets and peripheral CD4⁺ T cells. Frequencies of anergic CD4⁺ T cells (CD73⁺FR4⁺) were compared across genotypes. Functional signaling was assessed via calcium flux and phospho-flow (pERK, pS6) after stimulation.
Results: Nur77-eGFP expression track with TCR signal strength and reveal genotype-dependent differences in self-reactivity (Fig. 1). In GFPlo thymocytes and peripheral naïve CD4⁺ T cells, mean fluorescence intensity (MFI) scaled with ZAP70 function: WT > YYAA > SKG. In contrast, GFPhi CD4⁺ T cells were more abundant in SKG, likely reflecting a more self-reactive repertoire (Fig. 2). Consistent with prior self-antigen encounter, GFPhi CD4 single-positive thymocytes and peripheral CD4⁺ T cells in SKG and YYAA mice showed dampened signaling upon restimulation, including reduced calcium flux and pERK/S6. Repertoire analysis further supported increased self-reactivity in SKG: TCR Vβ chains recognizing endogenous retroviral superantigens—typically deleted in WT mice—escape deletion in SKG and, to a lesser extent, YYAA. This suggests impaired TCR signaling compromises central tolerance and permits expansion of autoreactive clones. YYAA retain similar or higher frequencies of CD73⁺FR4⁺ anergic CD4⁺ T cells than SKG. Although GFPhi CD4⁺ T cells from all genotypes upregulate inhibitory receptors (CD5, PD-1, LAG3, TIM-3, FR4), SKG show reduced expression of CD5, PD-1, and FR4 following peripheral self-antigen encounter. In contrast, YYAA more effectively induced these pathways.
Conclusion: TCR signaling strength controls the balance between deletion, anergy, and activation in developing and peripheral CD4⁺ T cells. Hypomorphic ZAP70 mutations impair thymic selection—altering the TCR repertoire—and peripheral tolerance. Our findings suggest anergy may serve as an additional central tolerance mechanism when deletion is inefficient. In SKG, defective peripheral tolerance permits self-reactive CD4⁺ T cell activation and arthritis. In contrast, less impaired anergy and tolerance pathways in YYAA may restrain autoreactive T cells and prevent disease despite RF autoantibody production. These findings highlight a TCR signaling threshold that governs tolerance enforcement and autoimmune risk.
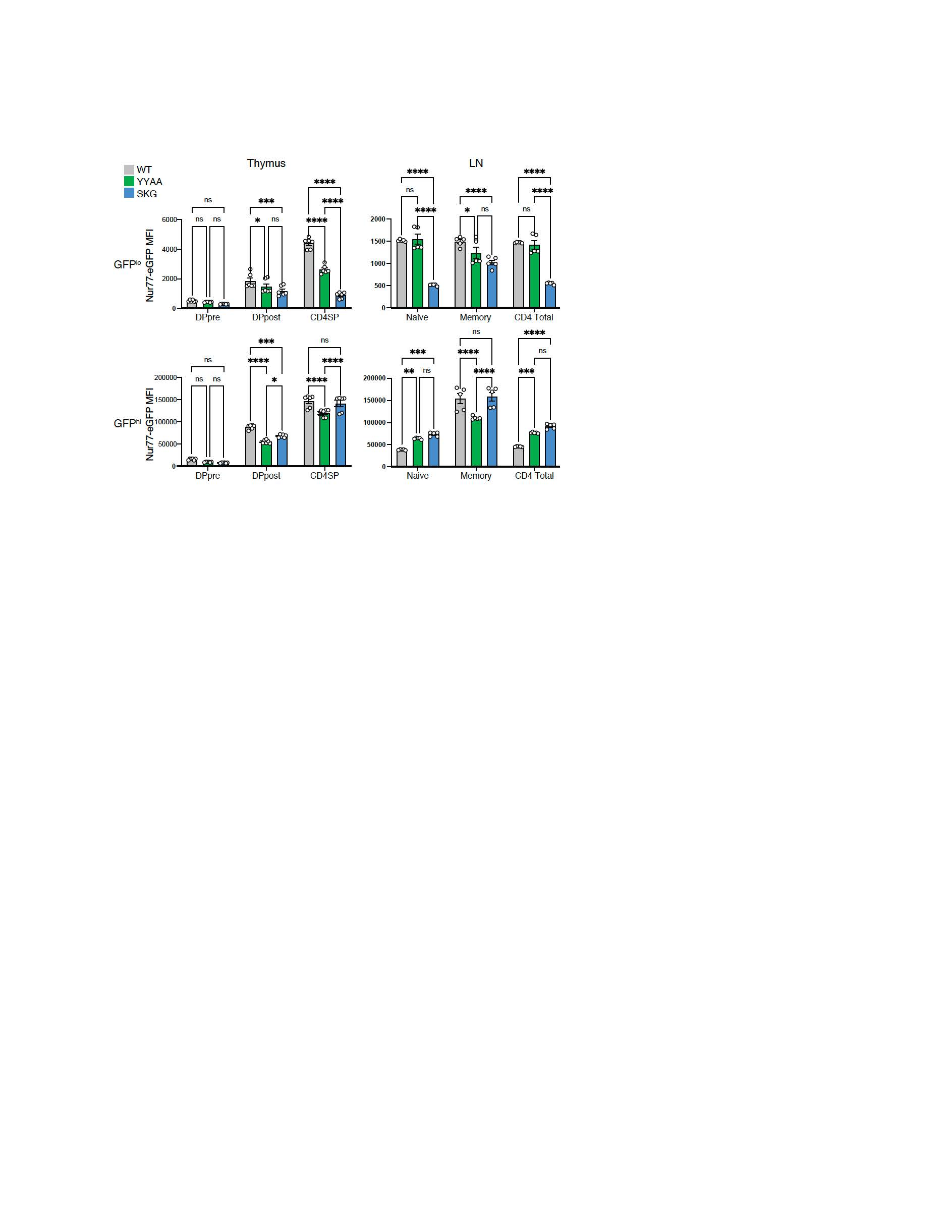 Figure 4. Nur77-eGFP and CD5 expression reveal genotype-dependent differences in TCR signaling across thymic and peripheral CD4⁺ T cell subsets.
Figure 4. Nur77-eGFP and CD5 expression reveal genotype-dependent differences in TCR signaling across thymic and peripheral CD4⁺ T cell subsets.
Nur77-eGFP serves as a cumulative readout of integrated TCR signaling, while CD5 reflects proximal TCR signal strength. Histograms show expression of Nur77-eGFP (top) and CD5 (bottom) in pre-selection (DP pre; CD69loTCRβlo) and post-selection (DP post; CD69hiTCRβhi) double-positive thymocytes, CD4 single-positive thymocytes (CD4+TCRβpos), peripheral conventional CD4⁺CD25⁻ T cells (CD4 conv), and CD4⁺CD25⁺ regulatory T cells (Treg) from WTNur (black), YYAANur (green), and SKGNur (blue) mice. Data are representative of ≥ 2 independent experiments with 3 mice per genotype.
.jpg) Figure 2. Nur77-eGFP expression in SKG peripheral T cells reflects their increased self-reactivity.
Figure 2. Nur77-eGFP expression in SKG peripheral T cells reflects their increased self-reactivity.
Mean fluorescence intensity (MFI) of Nur77-eGFP was quantified in GFPlo (top panels) and GFPhi (bottom panels) thymocyte subsets (left panels; n = 6/genotype) and peripheral CD4⁺CD25⁻ T cells from lymph nodes (right panels; n = 5/genotype) in WTNur (gray), YYAANur (green), and SKGNur (blue) mice. GFPlo cells reflect basal/tonic TCR signaling, while GFPhi cells represent cells with higher self-reactivity. In the thymus, GFPlo MFI scales with ZAP70 function (WT > YYAA > SKG), whereas in the periphery, SKG mice exhibit increased GFPhi cells. Statistical significance determined by two-way ANOVA with multiple comparisons. ns: P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Data pooled from two independent experiments.
To cite this abstract in AMA style:
Nakao Y, Patel A, Yang L, yu S, Weiss A, Ashouri J. TCR Signaling Thresholds Govern Anergy and Tolerance in ZAP70 Hypomorphic Models of Autoimmune Arthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/tcr-signaling-thresholds-govern-anergy-and-tolerance-in-zap70-hypomorphic-models-of-autoimmune-arthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tcr-signaling-thresholds-govern-anergy-and-tolerance-in-zap70-hypomorphic-models-of-autoimmune-arthritis/
