Session Information
Date: Monday, November 18, 2024
Title: Systemic Sclerosis & Related Disorders – Basic Science Poster II
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Macrophages play a major role in dcSSc-related skin fibrosis, with a mixed M1-M2 activation profile relying on the activation of JAK/STAT. Tofacitinib, a pan-JAK inhibitor, down-regulates interferon (IFN) pathways in human fibroblasts, and modulates macrophage activation profile in SSc mouse models. Its impact on human skin macrophages in dcSSc has not been studied. The objectives of our study were to identify skin macrophage subpopulations overrepresented in early dcSSc, and to assess the impact of tofacitinib on these subsets utilizing skin biopsies from a randomized controlled phase I/II trial (RCT: NCT03274076).
Methods: Single cell RNA sequencing (scRNAseq) of skin biopsies from 14 healthy donors (HD) were compared to skin biopsies from 26 early dcSSc to identify overrepresented macrophages subpopulations in dcSSc. In addition, data from a phase I/II RCT were utilized to evaluate the impact of tofacitinib on identified macrophage subpopulations: skin biopsies of 10 dcSSc patients receiving tofacitinib were assessed at baseline and at week 6 and were compared to skin biopsies from 5 dcSSc patients receiving placebo using scRNAseq. Impact on identified markers was secondly confirmed using qPCR in M-CSF monocytes-derived-macrophages (MDM) from HD treated in vitro by tofacitinib (0,1 and 1 μM for 24 hours) in resting conditions or after activation by IL4/IL13.
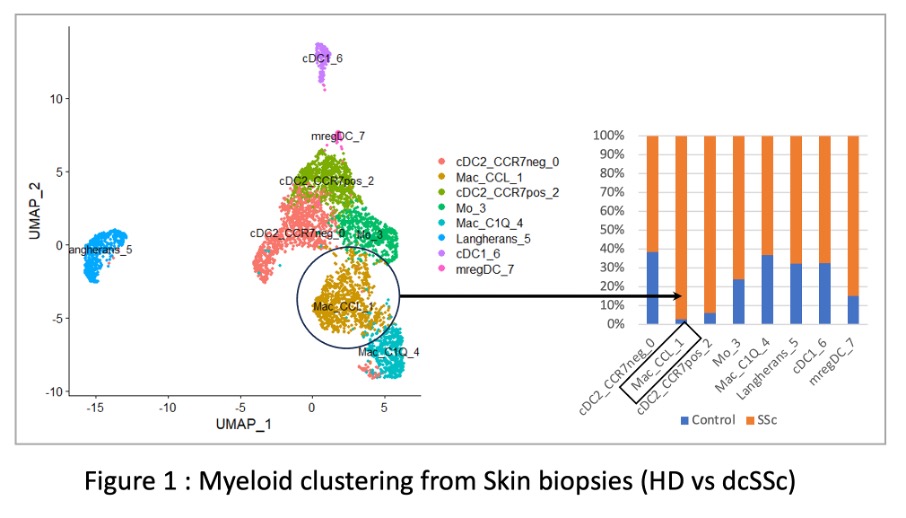
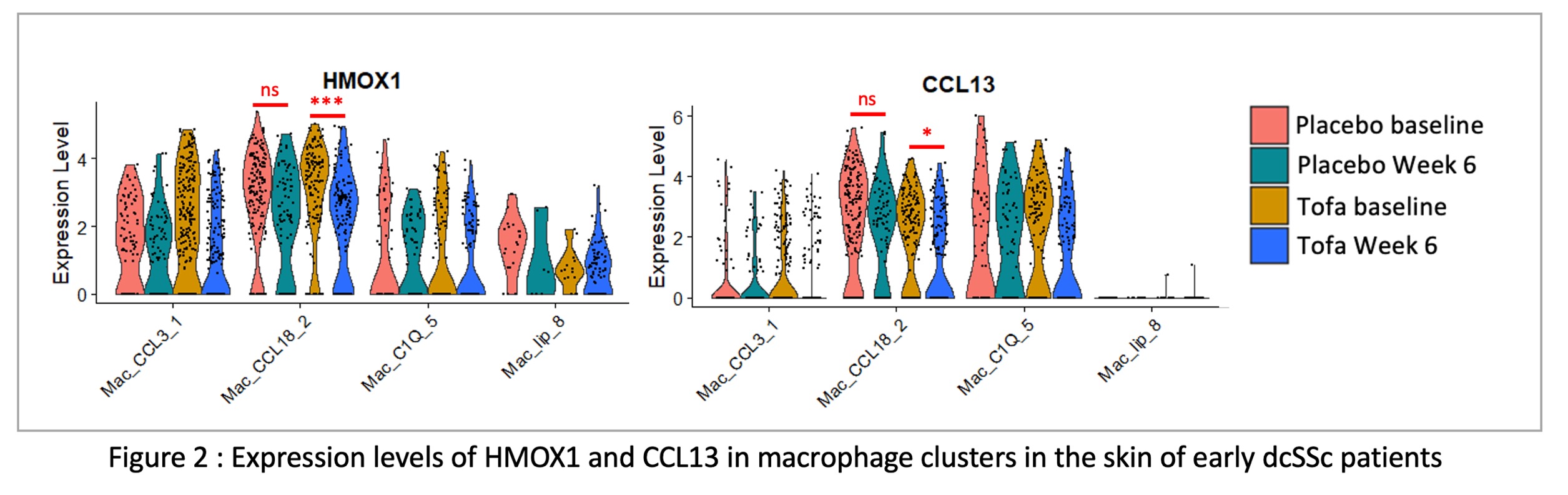
Results: Seven clusters of myeloid cells (1 Langerhans cell cluster, 4 dendritic cell clusters, 1 monocyte cluster and 2 macrophage clusters) were identified in skin biopsies by scRNAseq (Figure 1). Macrophage cluster 1 and CCR7-positive conventional DC were overrepresented in the skin of dcSSc patients as compared to HD. Macrophage cluster 1 was characterized by the expression of CCL18, HMOX1 and CCL13. Top 100 upregulated genes in this cluster were associated with pathways involved in fibrosis (e.g. Lung fibrosis-related pathways, TGFb signaling, IL-18 and IL-10 pathways). In the RCT, HMOX1 and CCL13 were both significantly downregulated by tofacitinib (Figure 2) whereas no significant downregulation was observed in the placebo group. HMOX1 expression at baseline in this macrophage cluster also correlated with JAK/STAT-dependent IL4/IL13 pathways. In vitro treatment of MDM by tofacitinib confirmed the significant downregulation of CCL13 and HMOX1 by tofacitinib in a dose-dependent manner.
Conclusion: Using scRNAseq, we identified a cluster of macrophages overrepresented in the skin of patients with early dcSSc and characterized by the expression of CCL13 and HMOX1, which were down-regulated by tofacitinib in patients with dcSSc from a phase I/II RCT. These results suggest that beyond its impact on IFN in fibroblasts, tofacitinib also represses genes associated with dcSSc-related macrophage activation profile, confirming a potential overall effect of this pan-JAK inhibitor on key SSc-related pathogenic mechanisms.
To cite this abstract in AMA style:
Ferrant J, Lescoat A, Lecureur V, Lelong M, Varga J, Lafyatis R, Gudjonsson J, Khanna D. Skin Macrophage Subtypes and Impact of Tofacitinib in Early Diffuse Cutaneous Systemic Sclerosis: Results from Single-cell Analyses of an Observational Data Set and a Phase I/II Randomized Controlled Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/skin-macrophage-subtypes-and-impact-of-tofacitinib-in-early-diffuse-cutaneous-systemic-sclerosis-results-from-single-cell-analyses-of-an-observational-data-set-and-a-phase-i-ii-randomized-controlled/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/skin-macrophage-subtypes-and-impact-of-tofacitinib-in-early-diffuse-cutaneous-systemic-sclerosis-results-from-single-cell-analyses-of-an-observational-data-set-and-a-phase-i-ii-randomized-controlled/