Session Information
Date: Sunday, November 17, 2024
Title: Innate Immunity Poster
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Recent studies have highlighted the importance of malondialdehyde-acetaldehyde (MAA)-modified proteins and resulting immune responses in the pathogenesis of rheumatoid arthritis (RA). MAA adducts have been detected in both joint and lung tissues of patients with RA. Moreover, this post-translational modification acts as a potent hapten, triggering systemic autoimmune and pro-fibrotic responses. Whether immune responses to MAA might also influence the development of other disease-related manifestations in RA remains unknown. With a well-recognized increase in the incidence of myocardial dysfunction leading to heart failure (HF) in RA, the aim of this study was to examine whether immunization with a MAA-modified protein induces myocardial deposition of extracellular matrix protein and evidence of tissue fibrosis.
Methods: Arthritis prone male DBA/1J mice (n=3/group) were given weekly intraperitoneal injections for 5 weeks of: 1) 25 μg/mL of unmodified vimentin (VIM), 2) 25 μg/mL of VIM-MAA, or 3) equal volumes of saline (additional negative control). Mice were sacrificed at week six. Heart tissues were resected, paraffin embedded, sectioned, and subjected to immunohistochemistry (IHC) using anti-MAA, anti-citrulline (CIT), and anti-VIM antibodies. Additional tissues were stained with trichrome for total collagen deposition and mice were assessed for arthritis development using a semi-quantitative score. Statistical analyses were performed using one-way ANOVA with Tukey’s multiple comparisons test.
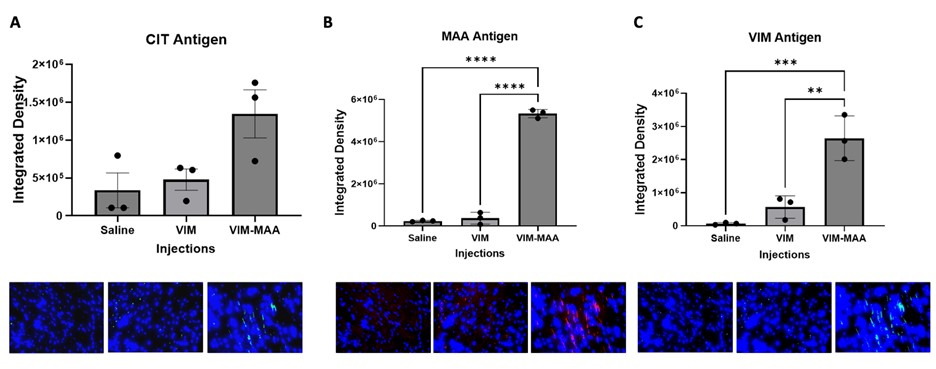
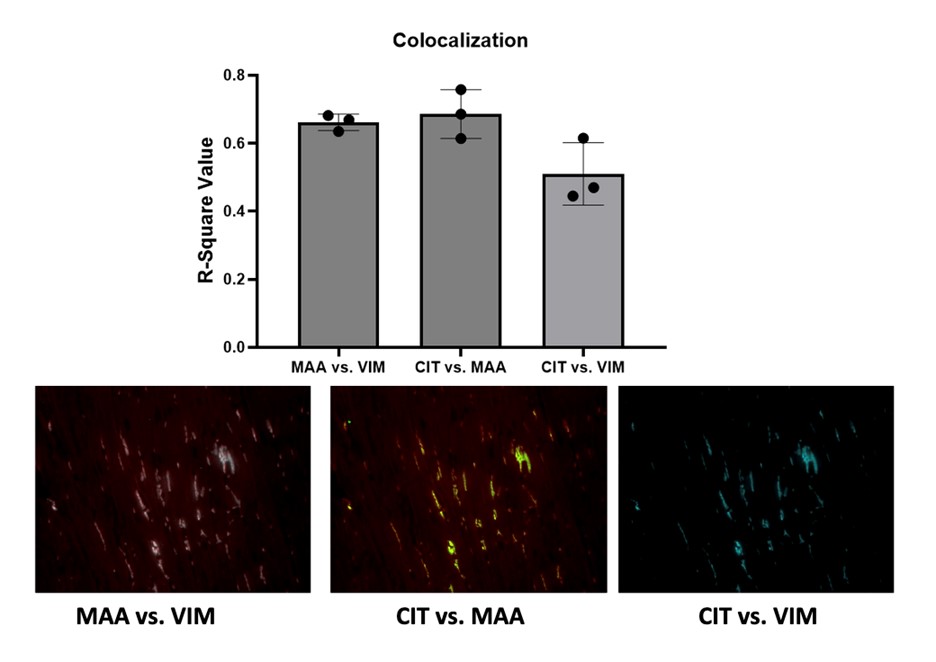
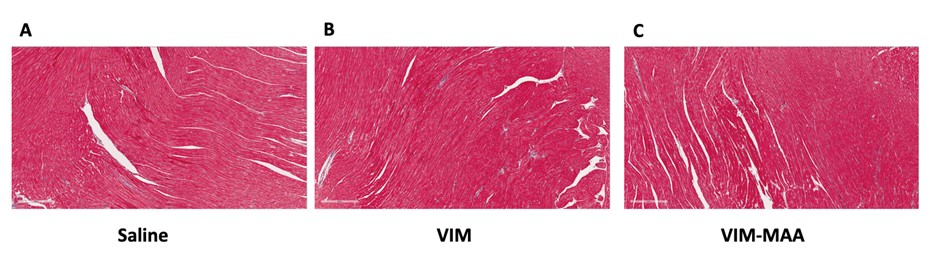
Results: IHC staining demonstrated the highest expression of MAA (p< 0.0001) and VIM (p< 0.01) antigens in heart tissues of VIM-MAA immunized mice (Fig. 1). Similar trends were observed for CIT, although these differences did not reach significance. In addition, there was a strong co-localization between MAA-CIT (r2 = 0.69), MAA-VIM (r2 = 0.66), and CIT-VIM (r2 = 0.51) (Fig. 2). However, trichrome staining of hearts revealed negligible evidence of collagen deposition in the absence of group differences (Fig. 3). No differences in arthritis scores were observed across the groups (data not shown).
Conclusion: This study is the first to demonstrate that systemic immunity to MAA-modified vimentin directly contributes to an increased expression of MAA and CIT modified proteins in heart tissues. Using this early arthritis model, however, evidence of tissue fibrosis was not observed. Additional investigations that include repeated immunizations and longer follow up will be needed to identify whether MAA immunization leads to myocardial dysfunction and evidence of local tissue inflammation and/or fibrosis.
To cite this abstract in AMA style:
Sinanan K, Zhou W, Duryee M, Aripova N, Poole J, Hunter C, Nelson A, Johnson T, Anderson D, Mikuls T, Thiele G. Immunization of Arthritis Prone Mice with Malondialdehyde-Acetaldehyde Modified Vimentin Induces Post-Translational Protein Modifications and Extracellular Matrix Deposition in Heart Tissues [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/immunization-of-arthritis-prone-mice-with-malondialdehyde-acetaldehyde-modified-vimentin-induces-post-translational-protein-modifications-and-extracellular-matrix-deposition-in-heart-tissues/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/immunization-of-arthritis-prone-mice-with-malondialdehyde-acetaldehyde-modified-vimentin-induces-post-translational-protein-modifications-and-extracellular-matrix-deposition-in-heart-tissues/