Session Information
Date: Monday, November 14, 2022
Title: T Cell Biology and Targets in Autoimmune and Inflammatory Disease Poster
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Histidyl-tRNA synthetase (HRS) is a key target of antigen-specific B and T cell responses in the anti-synthetase syndrome. Despite a clear role for aberrant adaptive immune responses in this disorder, accumulating evidence indicates that components of the innate immune system are also key regulators of the disease process—including macrophages that are a prominent component of unique perimyseal infiltrates found in the anti-synthetase syndrome. We therefore sought to determine the relative contribution of T cells and macrophages in our established model of HRS-induced myositis through phenotypic analysis of selected transgenic and knockout (KO) mice.
Methods: We used a recombinant protein consisting of the amino-terminal 151 amino acids of murine HRS fused to Maltose Binding Protein (MA/MBP) to immunize mice and induce myositis according to our previously published protocol. 17-21 days post IM immunization, we collected muscle tissue, lymph nodes, spleen, and serum from the following C57BL/6-derived strains: WT, OT-II TCR transgenic (OVA-specific TCR), RAG1 KO, CD4 KO, and CD4-Cre.MyD88fl/fl as well as Lyz2-Cre.MyD88fl/fl conditional KO mice. Muscle tissue was then processed for histopathological studies that included immunohistochemical staining for CD3, CD4, CD8, CD45, and CD68. Single cell isolation through physical and biochemical dissociation of muscle tissue was performed to facilitate flow cytometric assessment and single cell RNA sequencing (scRNAseq) of muscle-infiltrating mononuclear cells.
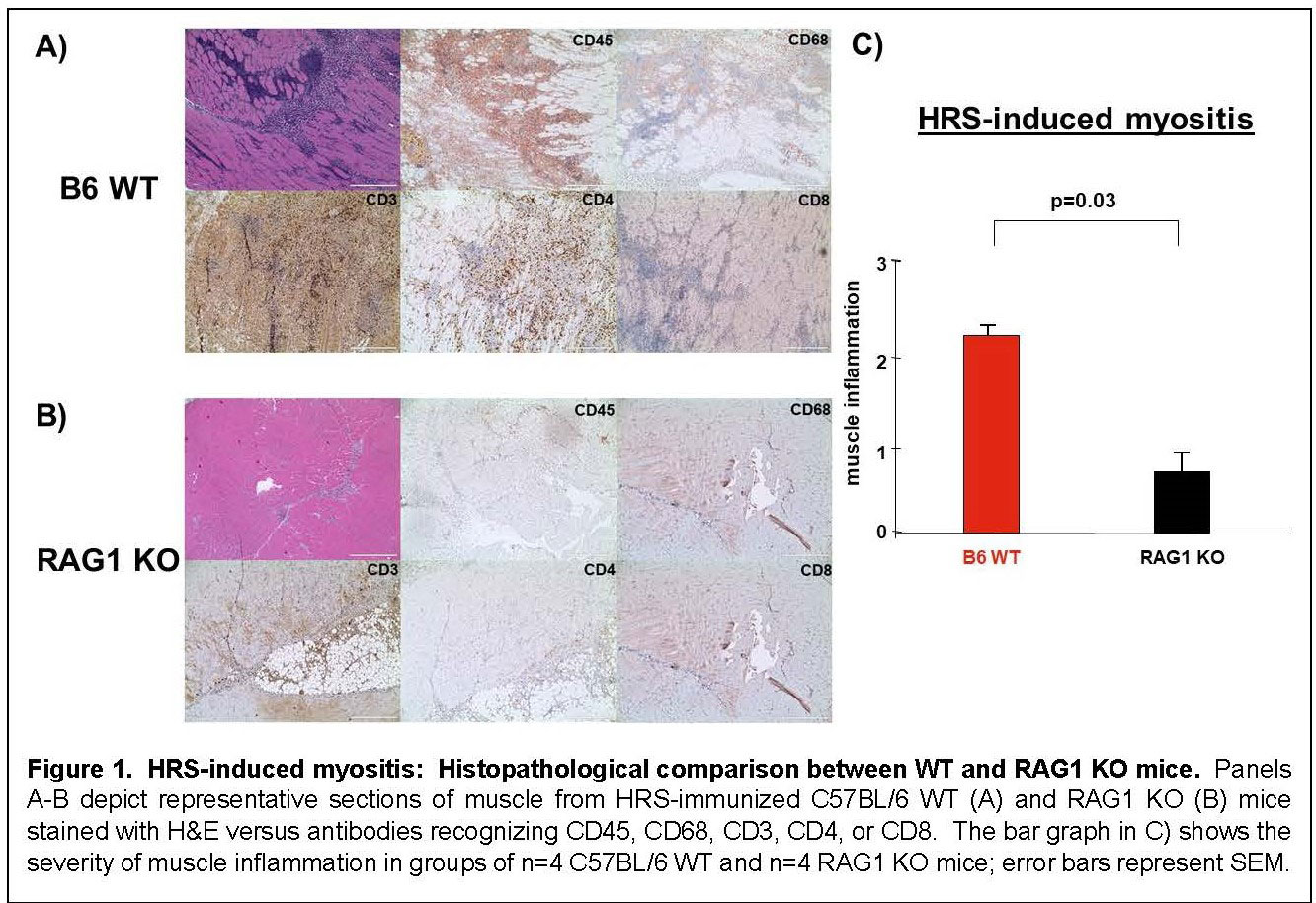
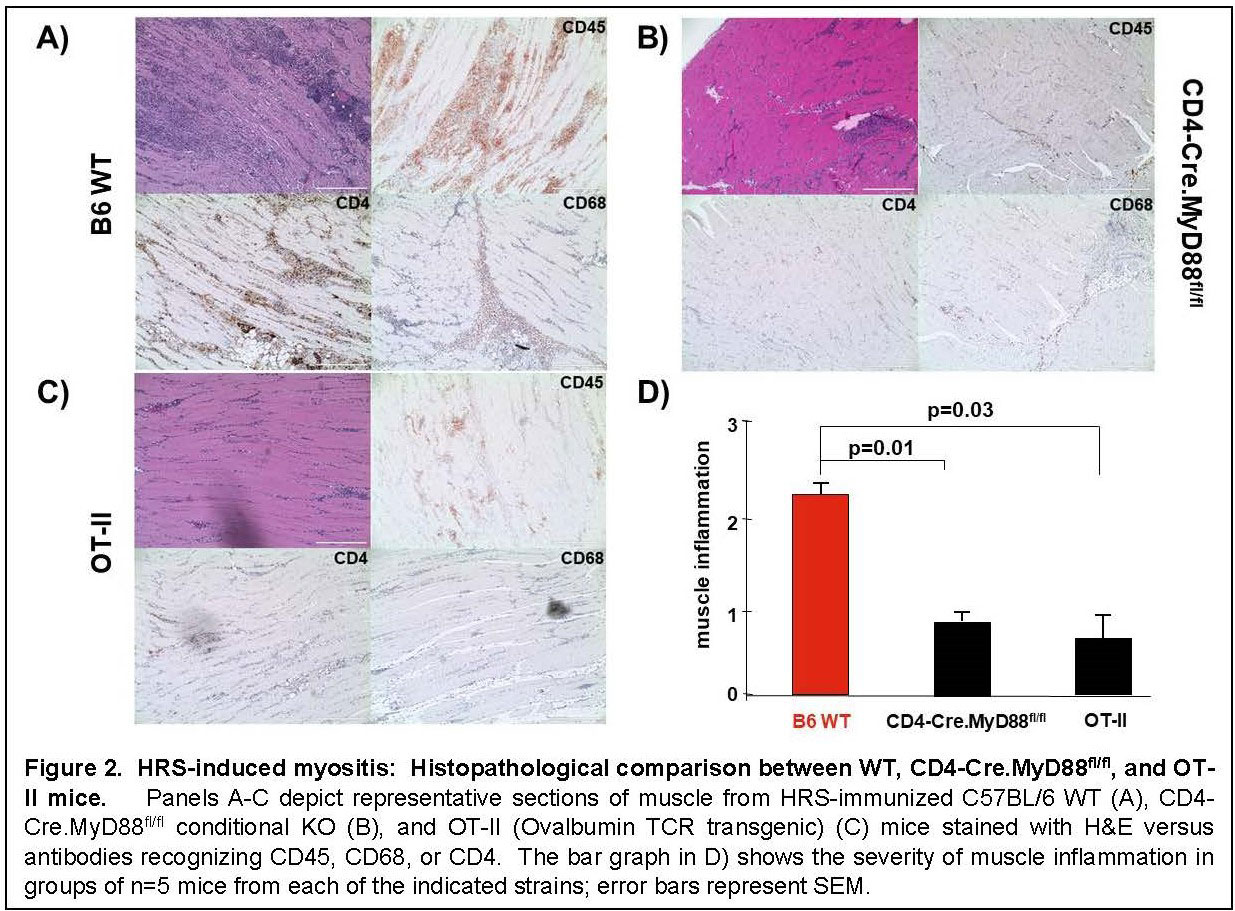
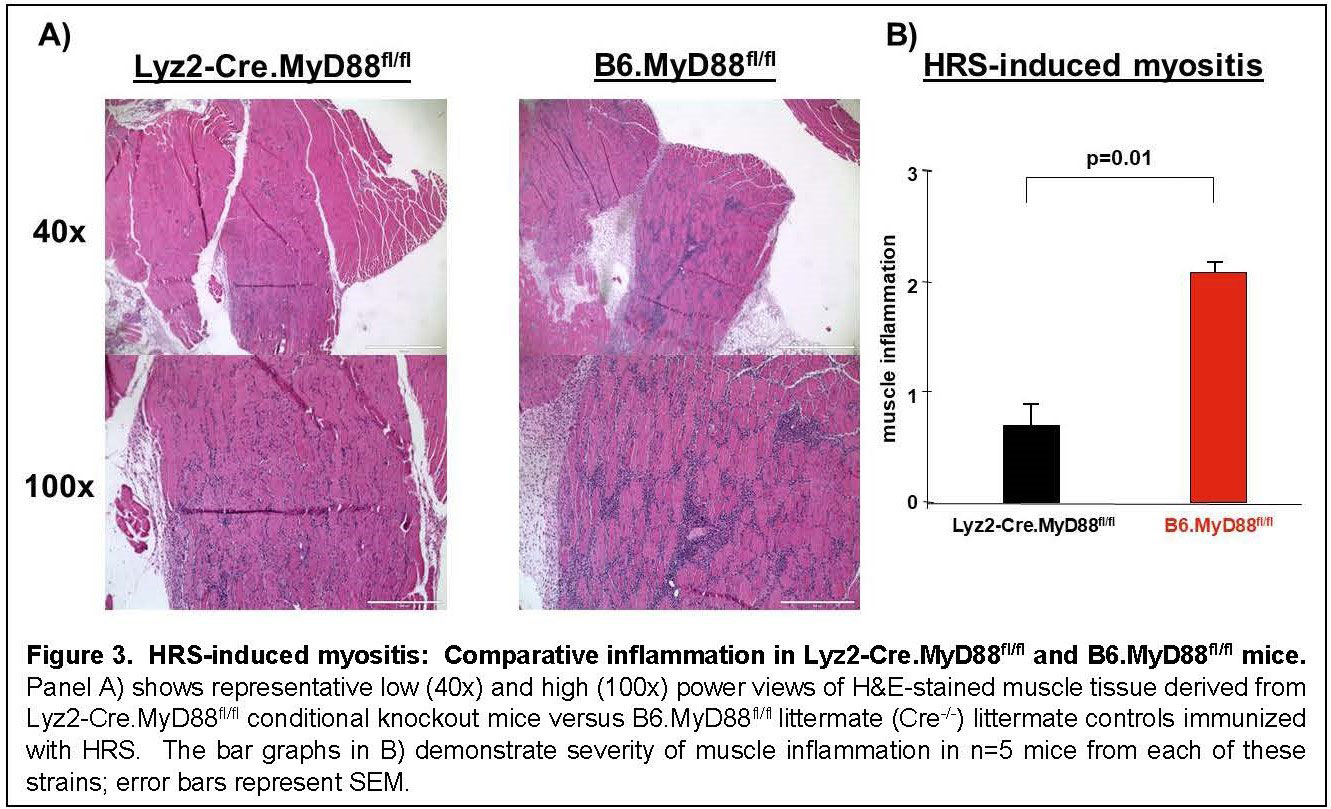
Results: Immunization of WT mice generated robust muscle inflammation consisting of CD4+ T cells, B cells, macrophages, and NK cells distributed in the perimysium and endomysium. The degree of inflammation was significantly reduced in RAG1 KO mice lacking functional BCR/TCR rearrangement, demonstrating an absolute requirement for T cells to attain full expression of HRS-induced myositis (Fig 1). While significant reduction of muscle inflammation in CD4 KO mice confirmed the importance of T cells in this model, parallel reduction of muscle-infiltrating lymphocytes in CD4-Cre.MyD88fl/fl conditional KO mice indicated that T cell activation/infiltration is at least partially dependent on MyD88-mediated innate immune signaling pathways. The limited myositis phenotype observed in OVA TCR transgenic mice demonstrated that antigen-specific recognition of HRS is also required for full expression of HRS-induced myositis (Fig 2). Virtual abrogation of muscle-infiltrating cells in Lyz2-Cre.MyD88fl/fl conditional KO mice indicated that macrophages are needed to support both innate and adaptive immune activation of T cells in HRS-induced myositis (Fig 3). Finally, comparative scRNAseq analysis of macrophage subpopulations in WT, OT-II, CD4-Cre.MyD88fl/fl conditional KO, and RAG1 KO mice signified that T cell activation strongly impacts the gene expression profile and resulting cellular phenotype of muscle-infiltrating macrophages.
Conclusion: T cell-macrophage interactions dictate the phenotype of HRS-induced myositis, demonstrating a key role for both innate and adaptive immunity in this model of the anti-synthetase syndrome.
To cite this abstract in AMA style:
Reay D, Wang Y, Jarjour W, Clemens P, Ascherman D. T Cell-Macrophage Interactions Play a Critical Role in a Mouse Model of Histidyl-tRNA Synthetase-Induced Myositis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/t-cell-macrophage-interactions-play-a-critical-role-in-a-mouse-model-of-histidyl-trna-synthetase-induced-myositis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/t-cell-macrophage-interactions-play-a-critical-role-in-a-mouse-model-of-histidyl-trna-synthetase-induced-myositis/