Session Information
Date: Tuesday, November 9, 2021
Title: T Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster (1507–1515)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Current therapies for autoimmune and inflammatory diseases generally target select disease nodes, often failing to produce durable clinical remission. Chronic inflammation is associated with alterations in immune cell metabolism, and it is possible that modulating metabolism may result in more durable responses. Mucosa-associated lymphoid tissue lymphoma translocation protein-1 (MALT1) is a component of the CBM (CARMA/CARD-Bcl10-MALT1) signaling complex that plays a critical role in effector functions of multiple immune cell subsets implicated in autoimmune diseases. While MALT1 is known to regulate NF-kB activation, its role in metabolic reprogramming of immune cells has remained relatively unexplored. We have used allosteric small molecule inhibitors of MALT1 as tools to evaluate the relationship of MALT1 inhibition to activation-induced metabolic reprogramming with the goal of advancing MALT1 inhibitors as therapeutics for autoimmune disease.
Methods: Activated human memory T-cells, B-cells, macrophages, and whole blood were evaluated for the effect of MALT1 inhibitor on effector cell functions downstream of activation receptors. In vivo effects of MALT1 inhibition were evaluated in an anti-CD3 antibody treated mouse model of acute inflammation. Three rodent models of disease (EAE, chronic GVHD and CIA) were employed to evaluate effects of orally administered MALT1 inhibitors on disease activity and metabolite levels. Untargeted metabolomics was performed via LCMS in human memory T-cells and tissues from animal studies.
Results: We identified a common set of metabolite changes that are conserved across multiple immune cell subsets activated via immune tyrosine activated motif (ITAM) containing receptors. This metabolite-based ‘activation signature’ was reversed by MALT1 inhibition in vitro. In parallel, MALT1 inhibition also decreased proinflammatory cytokine production from the same sets of cells: TCR-activated memory T-cells and immune-complex stimulated macrophages, as well as BCR-induced B-cell proliferation. The metabolic activation signature was also observed in vivo in isolated splenocytes from anti-CD3 challenged mice and again was associated with decreased cytokine production. Treatment with MALT1 inhibitors led to disease improvement in a mouse model of sclerodermatous cGVHD, to decrease in disease scores in a TH17-driven EAE model, and to reversal of joint pathology in a rat CIA model. We found that the effect of MALT1 inhibition on disease score coincided with alterations in metabolite levels at sites of inflammation. These data suggest that MALT1-dependent metabolic reprogramming is associated with immune cell activation and disease activity and opens up a new avenue for evaluating the activity of therapeutic agents.
Conclusion: Our results identify MALT1-dependent effects on immune cell metabolism downstream of immune activation and associate those changes with disease activity. Ongoing studies are exploring 1) the role these MALT1-dependent changes in metabolism play in directing the effector function of disease-relevant immune cells and 2) whether metabolic changes may help identify important new biomarkers for disease-associated effector cells.
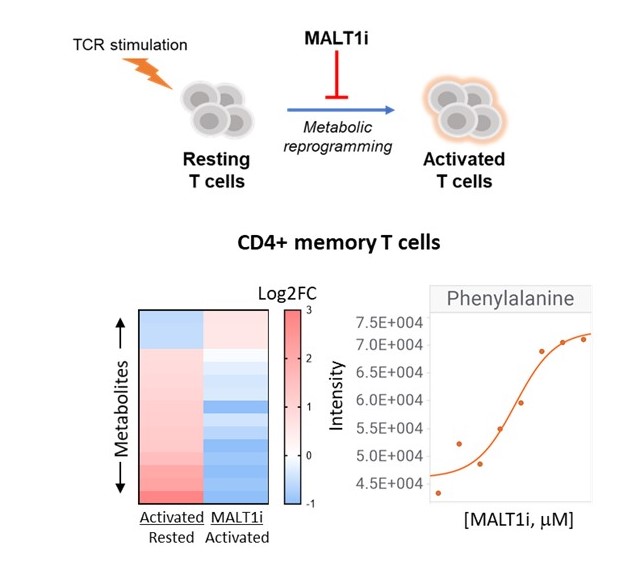 Figure. 1. Effect of MALT1 inhibition on metabolic reprogramming of T-cells. Metabolites were measured from human CD4+CD45RO+ cells 24 hours after stimulation with anti-CD3/CD28/CD2. MALT1 treated cells (MALTi) were preincubated with 5 uM REO-751 for 30 minutes. Dose-dependent effect (0-5 uM MALT1i) on essential amino acids (e.g., Phe) is shown.
Figure. 1. Effect of MALT1 inhibition on metabolic reprogramming of T-cells. Metabolites were measured from human CD4+CD45RO+ cells 24 hours after stimulation with anti-CD3/CD28/CD2. MALT1 treated cells (MALTi) were preincubated with 5 uM REO-751 for 30 minutes. Dose-dependent effect (0-5 uM MALT1i) on essential amino acids (e.g., Phe) is shown.
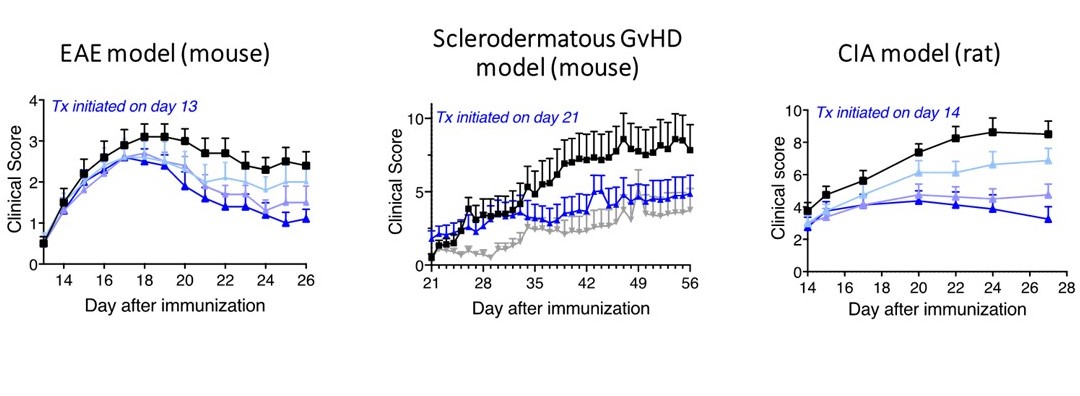 Figure 2. MALT1 inhibition is efficacious in multiple animal models of chronic inflammatory diseases. Black squares represent treatment with vehicle, blue triangles represent different doses (0.1 – 10 mg/kg; 100 mg/kg in scGVHD) of MALT1 inhibitor administered daily via oral route, gray inverted triangles represent Ruxolitinib, a JAK1/2 inhibitor as a comparator. n = 8_15 animals per group.
Figure 2. MALT1 inhibition is efficacious in multiple animal models of chronic inflammatory diseases. Black squares represent treatment with vehicle, blue triangles represent different doses (0.1 – 10 mg/kg; 100 mg/kg in scGVHD) of MALT1 inhibitor administered daily via oral route, gray inverted triangles represent Ruxolitinib, a JAK1/2 inhibitor as a comparator. n = 8_15 animals per group.
To cite this abstract in AMA style:
Biswas S, Steadman M, Helou Y, Sellers K, Soh K, Chalishazar A, Badur M, DiSpirito J, DeChristopher B, Monroe J, Rabah D, Fox B, Long A. Pharmacological Inhibition of MALT1 Reverses Activation-Induced Metabolic Reprogramming and Ameliorates Autoimmune Pathogenesis in Multiple Animal Models of Chronic Inflammation [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/pharmacological-inhibition-of-malt1-reverses-activation-induced-metabolic-reprogramming-and-ameliorates-autoimmune-pathogenesis-in-multiple-animal-models-of-chronic-inflammation/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pharmacological-inhibition-of-malt1-reverses-activation-induced-metabolic-reprogramming-and-ameliorates-autoimmune-pathogenesis-in-multiple-animal-models-of-chronic-inflammation/
