Session Information
Date: Sunday, November 7, 2021
Title: Abstracts: T Cell Biology & Targets in Autoimmune & Inflammatory Disease (0966–0969)
Session Type: Abstract Session
Session Time: 11:00AM-11:15AM
Background/Purpose: T cell-derived pro-inflammatory cytokines are a major driver of rheumatoid arthritis (RA) pathogenesis. While CD4 T cells have traditionally been assumed to be the main cytokine-producing T cells, CD8 T cells rival CD4 T cells in terms of both cell numbers and production of IFNγ and TNF. We found that the majority of CD8 T cells in synovial tissue and fluid are defined by high granzyme K (GzmK) expression and intermediate granzyme B (GzmB) expression, a pattern rare in blood. Here, we characterize the functions and differentiation of these tissue CD8 T cell subsets and describe the effects of GzmK on synovial fibroblasts.
Methods: Blood, synovial fluid, and synovial tissue were obtained from patients meeting the 2010 ACR criteria for RA. Single-cell RNA-seq data sets were generated by integrating new and publicly available data from synovial tissue and fluid from patients with RA and from blood from healthy controls. Synovial fibroblasts were stimulated with recombinant GzmK and/or cytokines for 24 hours prior to harvest of supernatants and RNA. Cytokine production was assessed by intracellular cytokine staining and flow cytometry or by enzyme-linked immunosorbent assay (ELISA). Gene expression changes were analyzed by RT-PCR or bulk RNA-sequencing (RNA-seq). In cross-disease analysis, GzmK and GzmB gene signatures were derived from RA synovial tissue data and applied to other tissue CD8 T cell profiles from publicly available scRNA-seq data.
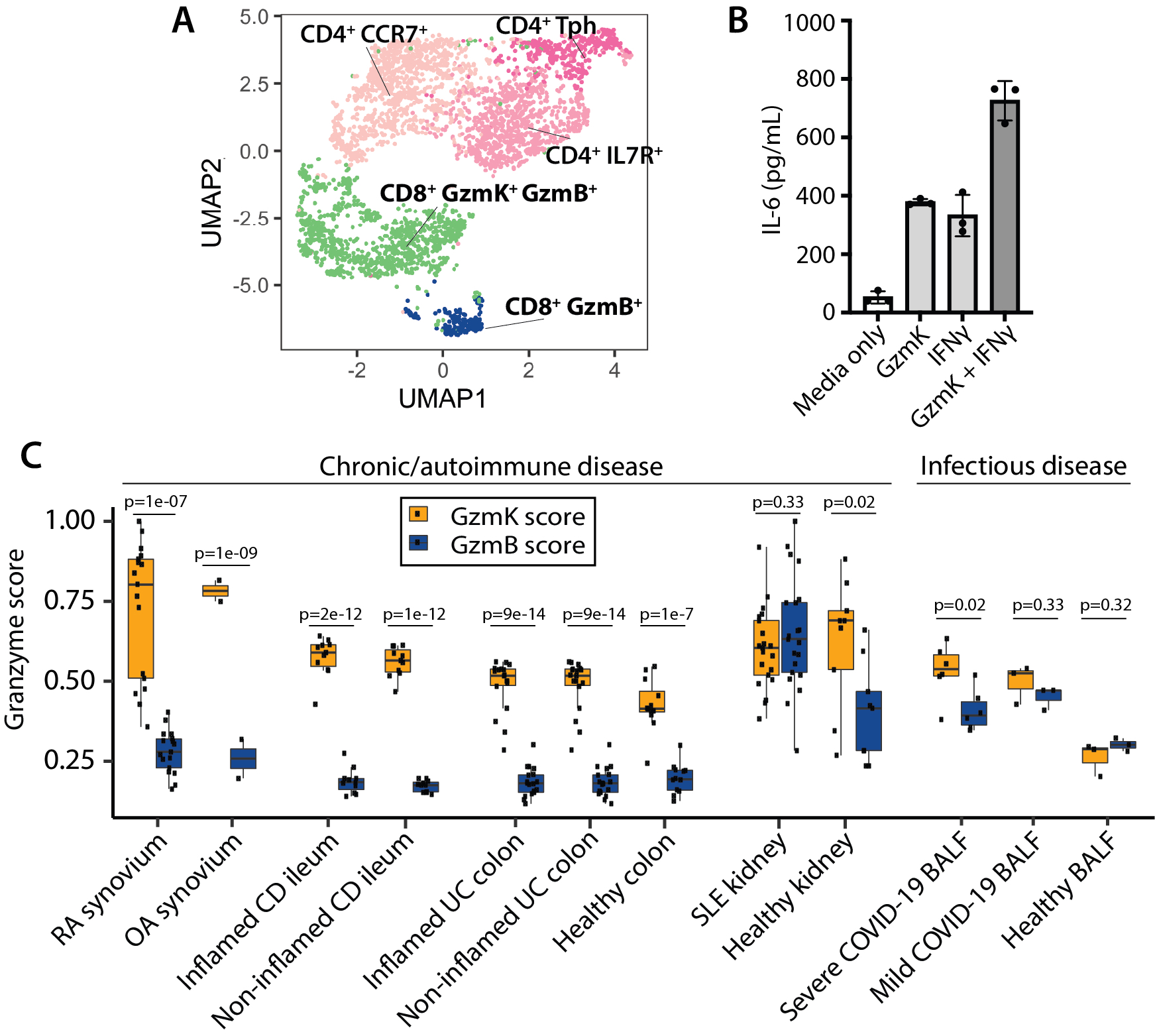
Results: The vast majority of synovial CD8 T cells have a phenotype that is distinct from classic cytotoxic T lymphocytes, which express high levels of cytotoxic proteins such as granzyme B (GzmB), perforin, and granulysin (Fig. 1A). Instead, synovial CD8 T cell populations are dominated by GzmK+ GzmB+ CD8 T cells with low expression of perforin. Unlike GzmB, which activated caspases to induce target cell death, we find that GzmK does not induce cell death of synovial fibroblasts. Instead, we find that GzmK induces synovial fibroblasts to produce inflammatory factors such as IL-6 and CCL2. Moreover, GzmK can potentiate the effects of IFNg and TNF on synovial fibroblasts, thereby amplifying the effects of these T cell-derived cytokines in RA synovium (Fig. 1B). Using single-cell TCR repertoire studies to track sister clones among matched blood and synovial CD8 T cells, we have identified migration and differentiation pathways that lead to the accumulation of GzmK+ GzmB+ CD8 T cells in inflamed joints. Further, we found transcriptomically similar CD8 T cells in both healthy and diseased tissues, including bowel from patients with inflammatory bowel disease and bronchoalveolar lavage fluid from patients with severe COVID-19 (Fig. 1C).
Conclusion: These findings suggest that GzmK+ GzmB+ CD8 T cells form a core population of tissue-associated T cells in humans that has the potential to drive inflammation both by production of inflammatory cytokines and by direct effects of granzyme K on other cells.
To cite this abstract in AMA style:
Jonsson A, Zhang F, Gomez-Rivas E, Watts G, Dunlap G, Faust H, Rupani K, Mears J, Rao D, Wang R, Keras G, Meednu N, Coblyn J, Massarotti E, Todd D, McDavid A, Anolik J, AMP: RA/SLE Network A, Wei K, Raychaudhuri S, Brenner M. Granzyme K+ CD8 T Cells Form the Core Population of Inflamed Human Tissue-associated CD8 T Cells [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/granzyme-k-cd8-t-cells-form-the-core-population-of-inflamed-human-tissue-associated-cd8-t-cells/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/granzyme-k-cd8-t-cells-form-the-core-population-of-inflamed-human-tissue-associated-cd8-t-cells/