Session Information
Session Type: Abstract Session
Session Time: 4:00PM-4:50PM
Background/Purpose: Abnormal vitamin D levels occur frequently in antiphospholipid (APS) patients and are correlated with thrombosis. It remains unclear however if vitamin D deficiency observed in APS occurs as a part of disease pathogenesis, as a consequence of disease activity or as an incidental disease-modifying factor. This study aims to determine if induction of antiphospholipid antibody (aPL) induces changes in vitamin D levels (Phase I) and to evaluate the effect of vitamin D deficiency on aPL-mediated thrombosis in APS mouse models (Phase II).
Methods: In Phase I, CD1 male mice (n= 5 to 7 per group) fed normal diets were immunized with either human β2GPI of varying doses (0.5ug, 1ug, 10ug or 150ug with adjuvant) once weekly for 3 doses to induce different levels of aPL activity or ovalbumin (OA) (10ug or 150ug). Serum was collected 4 weeks following initial immunization to measure 25 hydroxy vitamin D (25OHVD), aCL and anti-β2GPI levels by ELISA. In Phase II, CD1 male mice (n=5 to 7) were fed either a vitamin D normal (VDnorm) or deficient (VDdef) diet supplemented with calcium for 6 weeks until 25OHVD levels were stable. After stable 25OHVD levels, mice were then inoculated (2 doses over 48hrs) with either purified whole IgG from a primary APS (PAPS) patient (IgG APS 1), β2GPI-affinity purified IgG from a second PAPS patient (IgG APS 2) or whole IgG from normal human serum (IgG NHS) prior to thrombosis induction in the femoral vein. Blood was collected weekly after start of diet, immediately prior to IgG treatment and at time of thrombosis analysis.
Results: Phase I – IgG aCL and anti-β2PI levels were significantly higher in β2GPI immunized mice compared to control mice and increases were dependent on dose of antigen. However, there was no significant difference in 25OHVD levels among all the groups and all were normal (range 61.2±15.1 to 78.5±9.6 ng/ml, p=0.220). In phase II, VDdef mice developed vitamin D deficiency (< 20ng/ml) by W3, which continued until W6. Thrombus experiments were subsequently done at W4 and mean 25OHVD was significantly less in VDdef vs VDnorm mice (17.7±2.9 vs 36.1±7.8 ng/ml,p< 0.0001). Mean IgG anti-β2GPI levels were similar for VDdef vs VDnorm mice treated with IgG NHS (2.5±0.6 vs 2.8±0.4 G units), IgG APS 1 (75.9±0.2 vs 75.9±0.4) and IgG APS 2 (42.8±0.3 vs 42.3±0.3) at the time of surgery. Mean thrombus sizes in these 3 treatment groups were (555.9±89.5 vs 574.3±82.8 μm2,p=0.902), (2327.8±709.8 vs 1195.6±265.5,p< 0.001) and (5461.8±715.8 vs 2353.7±694.7,p< 0.001) for VDdef vs VDnorm mice (Figure 1).
Conclusion: We provide the first mechanistic data indicating that vitamin D deficiency amplifies the thrombogenic effect of IgG aPL. This effect was demonstrated for aPL derived from 2 different primary APS patients. However, induction of aPL activity of varying strength in an active immunization model seemingly had no effect on vitamin D production and storage in the face of normal dietary intake. Future studies will focus on the immunomodulatory changes underlying the effect of vitamin D on the thrombogenic capacity of aPL and mechanisms that lead to abnormal vitamin D levels in APS patients.
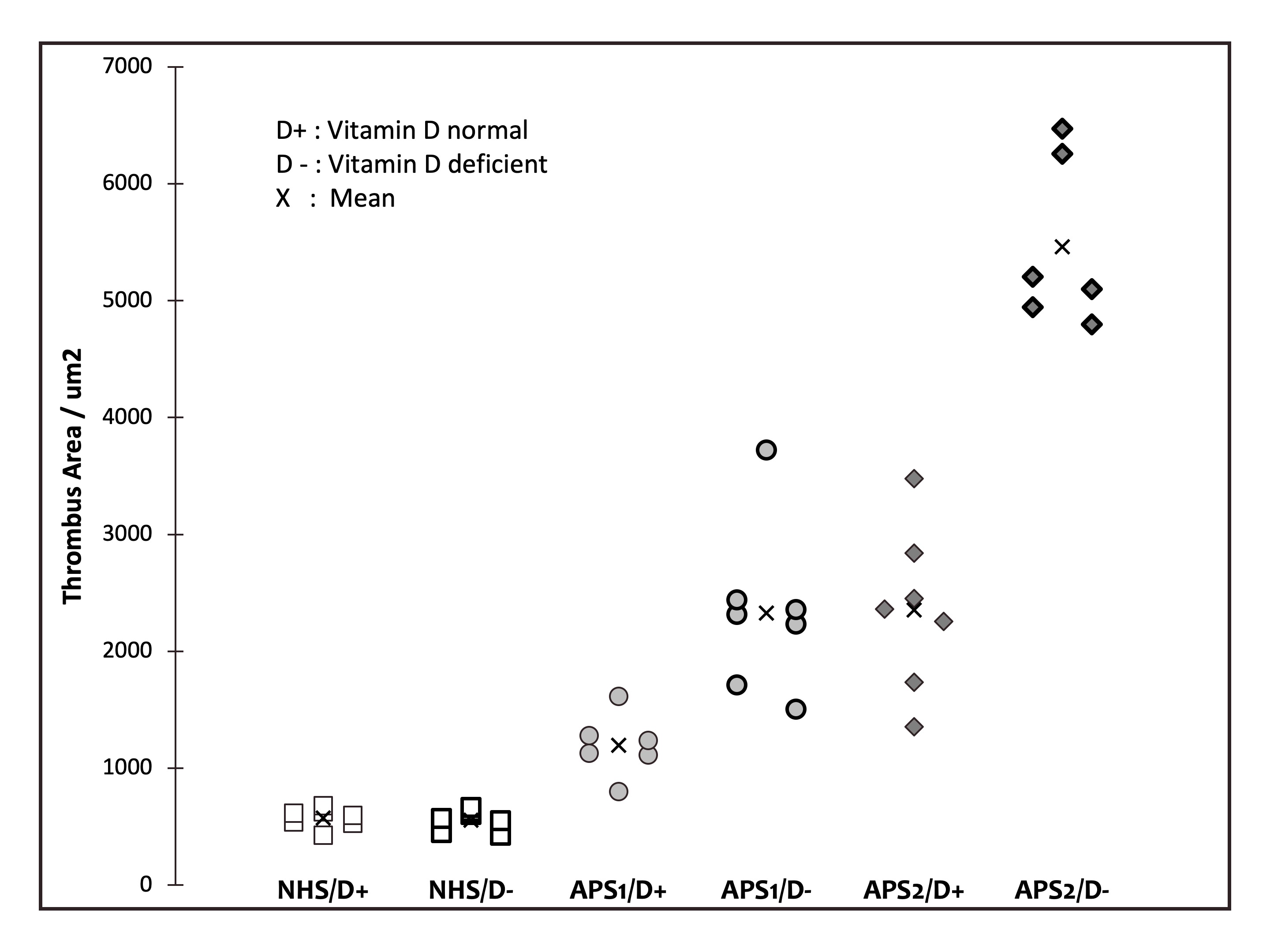 Figure 1. Cross-sectional area of induced thrombi in femoral vein of vitamin D deficient and normal mice treated with antiphospholipid and normal IgG
Figure 1. Cross-sectional area of induced thrombi in femoral vein of vitamin D deficient and normal mice treated with antiphospholipid and normal IgG
To cite this abstract in AMA style:
Willis R, Roye-Green K, Romay-Penabad Z, Papalardo E, Schleh A, Murthy V, Smikle M, Gonzalez E. Vitamin D Deficiency Enhances Antiphospholipid Antibody-Mediated Thrombosis in a Passive Immunization Mouse Model [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/vitamin-d-deficiency-enhances-antiphospholipid-antibody-mediated-thrombosis-in-a-passive-immunization-mouse-model/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/vitamin-d-deficiency-enhances-antiphospholipid-antibody-mediated-thrombosis-in-a-passive-immunization-mouse-model/
