Session Information
Session Type: Abstract Session
Session Time: 9:30AM-9:45AM
Background/Purpose: Rheumatoid arthritis (RA) is a chronic inflammatory joint disease that is female predominant. The TNF-transgenic (TNF-Tg) mouse model of RA also develops a sexually dimorphic inflammatory arthritis with earlier disease appearing in female mice (1). While the mechanism of the sexual dimorphism is unknown, studies suggest androgens can provide a protective effect against inflammatory disease progression (2,3). We hypothesize that androgens’ anti-inflammatory effects on myeloid cells in the joint may mediate inflammatory arthritis progression.
Methods: Orchiectomy and sham control procedures were performed on 4-week old male TNF-Tg mice (n=3 mice/group). Mice were sacrificed at 3-months old for serum and tissue collection. Testosterone and TNFɑ levels were compared between groups, and knee joints sections were H&E and TRAP stained for histological scoring. Bone marrow-derived macrophages (BMDMs), plated from 3-month old male and female TNF-Tg mice (n=3 mice/group), were treated with 0.1µM, 0.5µM, and 1µM of testosterone propionate (T), followed by murine IFNɣ and LPS stimulation. After 48 hours, supernatant was assayed in an ELISA for murine TNFɑ. Groups were analyzed using unpaired t-tests for orchiectomy studies and two-way ANOVA for in vitro studies. Values are reported as the mean ± standard deviation.
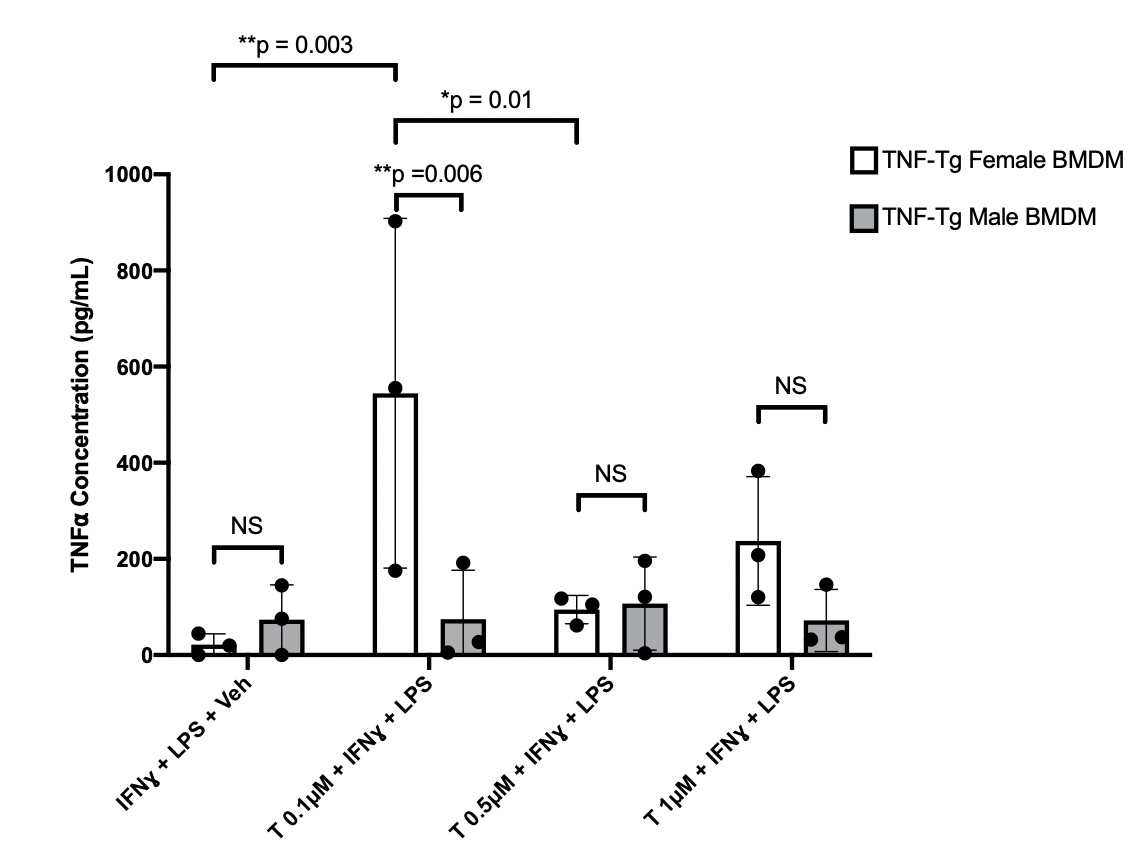
Results: Orchiectomized (Orch) mice joints had significantly higher (i.e. worse) histology scores than sham controls for synovial inflammatory infiltrate, pannus invasion, and TRAP+ area (2 Orch vs 1 Sham, 1.6 Orch vs 0.7 Sham, 1.4 Orch vs 0.3 Sham, p< 0.05). Orchiectomized TNF-Tg mice were confirmed to have low testosterone levels (0.078 ng/mL (0.054-0.12) ng/mL, normal=1.3 ± 0.4 ng/mL). Serum human (h) and mouse (m)TNFɑ levels remained unchanged between orchiectomized and sham TNF-Tg mice (827 pg/mL, range 271-1400 pg/mL Orch vs 841 pg/mL, range 613-1011 pg/mL Sham hTNF and 8.5 pg/mL, range 7-10 pg/mL Orch vs 8.8 pg/mL, range 8-9.8 pg/mL Sham mTNF). Male derived BMDMs did not have a significant change in TNFɑ expression when treated with T at any concentration compared to untreated cells (74.84 ± 101.9 pg/mL T 0.1µM, 107.1 ± 97.05 pg/mL T 0.5µM, 71.92 ± 64.55 pg/mL T 1µM vs 73.56 ± 72.48 pg/mL Untreated, p >0.05). Remarkably, female derived BMDMs had increased TNFɑ expression at a concentration of 0.1µM compared to untreated cells (544.06 ± 363.6 pg/mL T 0.1µM vs. 21.50 ± 22.48 pg/mL Untreated, p< 0.01) (Fig 1).
Conclusion: Here we show that reduced levels of testosterone from orchiectomies is associated with increased synovial inflammation in TNF-Tg males, yet does not affect systemic TNFɑ levels. In vitro studies of BMDMs indicate that macrophages from TNF-Tg mice have sex and testosterone concentration specific release of TNFɑ, suggesting a sexual dimorphism at the cellular level. Further trials of in vivo and in vitro T administration to both sexes is necessary to clarify local versus systemic immune responses in TNF-Tg mice.
1. Bell, R.D. et al. Arthritis Rheumatol 71(9):1512-1523. 2019.
2. Traish A et al. J Clin Med 7(12):549. 2018.
3. Lashkari M et al. Electron Physician 10(3):6500-6505. 2018.
To cite this abstract in AMA style:
Chen K, Lin X, Xing L, Kenney H, Bell R, Schwarz E, Rahimi H. Variable Effects of Testosterone on Male versus Female Derived Macrophages in Inflammatory Arthritis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/variable-effects-of-testosterone-on-male-versus-female-derived-macrophages-in-inflammatory-arthritis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/variable-effects-of-testosterone-on-male-versus-female-derived-macrophages-in-inflammatory-arthritis/