Session Information
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
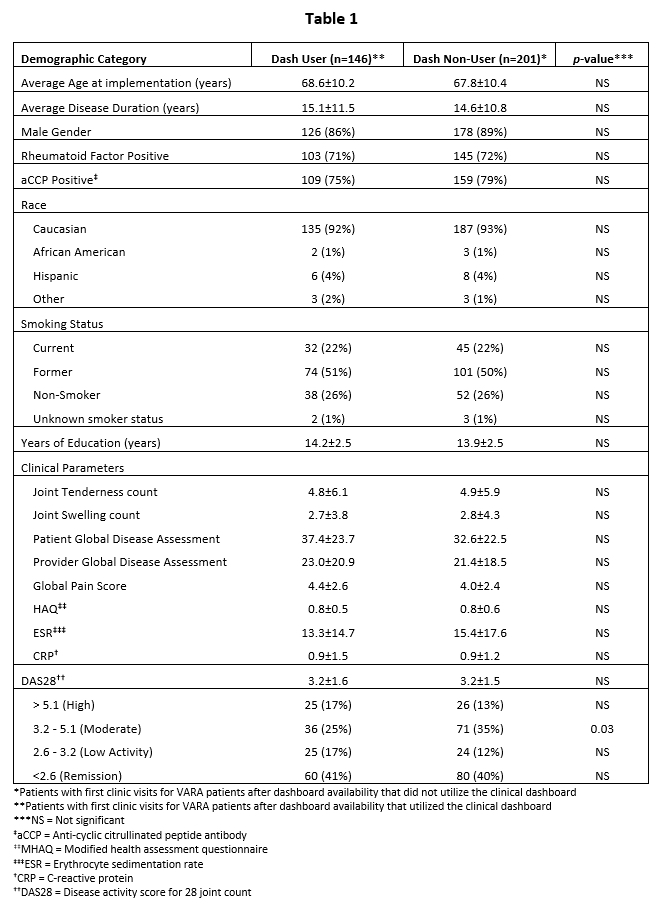
Background/Purpose: Rheumatoid Arthritis (RA) management guidelines recommend the collection of Disease Activity Measures (DAMs) to direct therapy as a component of a “treat-to-target” (T2T) strategy. A goal of the Veterans Affairs Rheumatoid Arthritis (VARA) registry is to systematically collect 6 clinical DAMs and two laboratory DAMs at each visit using note templates (Table 1). The real-time calculation of disease severity scores such as DAS28 is not part of the standard VARA protocol. We developed an on-line clinical dashboard that collects real-time DAMs and displays them longitudinally to calculate disease activity scores including DAS28 in real-time. In this study, we evaluated the association of clinical dashboard utilization with 1) DAS28 capture in the medical record and 2) the frequency of major changes in RA therapy.
Methods: Clinical notes from initial clinic visits for each US Veteran enrolled in VARA from a single site (between 10/2012 and 6/2018 – when the dashboard became available) were evaluated for documentation of 8 possible DAMs and whether major therapeutic changes (MTCs) were undertaken. We compared clinic notes for visits where the clinical dashboard was deployed for data collection with visits where the clinical dashboard was not used. MTC was defined as: initiation of synthetic or biologic disease-modifying antirheumatic drug (DMARD); DMARD dose escalation ≥25%; prednisone use (new agent or after 90-day gap); prednisone dose increased 25%; and/or corticosteroid injection in ≥2 joints. We also recorded changes and/or escalation in therapy even if it did not meet MTC criteria. Each MTC or lack of MTC was classified as being consistent or not consistent with ACR guidelines and our previously published potential optimal DAS28 threshold for MTC (See Table 2 Footnote).
Results: The 146 RA patients with a visit using the dashboard were similar to the 201 patients in whom the dashboard was not used referent to age, disease duration, clinical RA features, DAMs and overall DAS28 (Table 1). Clinic notes for patient visits using the dashboard were significantly more likely to record a DAS28 (75.3%) than visits not using the dashboard (2.0%) (p< 0.001) (Table 2). There was no significant difference in likelihood of an MTC, (20.5% vs 16.4%) or any therapeutic escalation (33.6% vs 27.4%) (Table 2). Management decisions to escalate therapy as recommended in ACR guidelines were more common at DAS28 >3.2 threshold for change (70.5% vs 60.2%, p=0.046) and DAS28 >3.8 threshold for change (77.4% vs. 66.7%, p=0.029).
Conclusion: Utilization of a clinical dashboard can improve documentation of disease activity scores such as DAS28 in real time. Further study is needed to determine if the use of a clinical dashboard will improve the implementation of care that is concordant with best practices and whether this leads to improvements in long-term patient outcomes.
To cite this abstract in AMA style:
Nelson T, Kunkel G, Cannon G. Use of a Clinical Dashboard Improves the Documentation of Disease Severity Scores and May Facilitate the Implementation of Treat to Target Therapy [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/use-of-a-clinical-dashboard-improves-the-documentation-of-disease-severity-scores-and-may-facilitate-the-implementation-of-treat-to-target-therapy/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-of-a-clinical-dashboard-improves-the-documentation-of-disease-severity-scores-and-may-facilitate-the-implementation-of-treat-to-target-therapy/