Session Information
Date: Saturday, November 7, 2020
Title: SLE – Treatment (0985–0989)
Session Type: Abstract Session
Session Time: 3:00PM-3:50PM
Background/Purpose: NOBILITY demonstrated improved renal responses and complete B-cell depletion with the humanized type II anti-CD20 monoclonal antibody obinutuzumab (OBI) compared with placebo (PBO) through 18 months in patients with proliferative lupus nephritis (LN) when added to standard of care therapies [1]. Two-year results from NOBILITY are reported here.
Methods: Patients with class III/IV LN received mycophenolate and steroids and were randomized to blinded OBI or PBO infusions on weeks 0, 2, 24, and 26. The primary endpoint was complete renal response (CRR) at week 52. Patients were followed through week 104. Secondary endpoints included overall renal response (ORR) and modified CRR (mCRR). The prespecified alpha level was 0.2 and analyses after week 52 were exploratory.
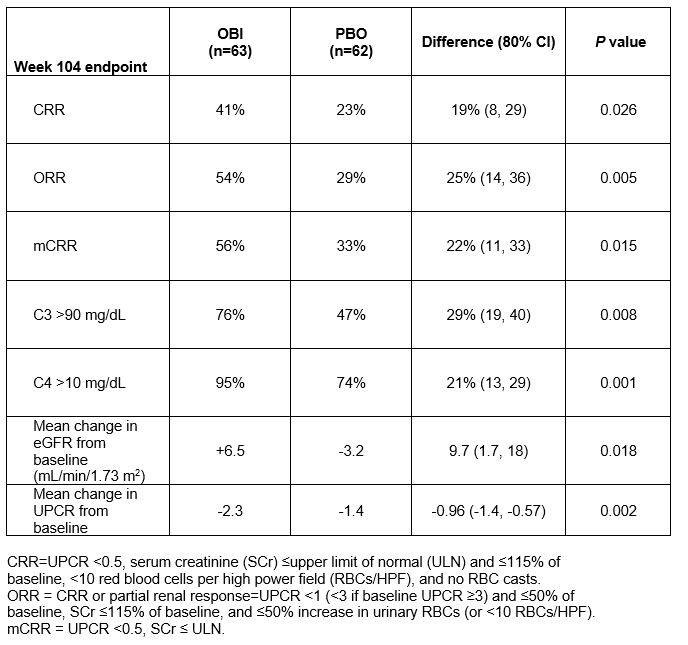
Results: 125 patients were randomized and received blinded infusions; 102 patients (82%) completed 104 weeks of follow up. CRR was greater with OBI than PBO at week 52 (35% vs. 23%, P = 0.115), week 76 (40% vs. 18%, P = 0.007), and week 104 (41% vs 23%, P = 0.026; Table). At week 104, OBI patients had greater improvement in eGFR, UPCR, anti-dsDNA, C3, and C4. Fewer OBI patients required initiation of a new rescue therapy through week 104 (OBI 6, PBO 12). Peripheral B-cells were detected in 92% of OBI patients at week 104. Serious adverse events (OBI 25% vs. PBO 30%), serious infections (8% vs. 18%) and deaths (1 vs. 4) were not increased with OBI.
Conclusion: NOBILITY demonstrated a sustained benefit of OBI through week 104, approximately 18 months after the final OBI infusion. The OBI dosing regimen was associated with a return of peripheral B-cells by week 104. There were no unexpected safety findings. OBI use in LN will be further evaluated in the Phase 3 REGENCY trial.
References:
1. Rovin B, et al. ASN 2019 [abstract].
To cite this abstract in AMA style:
Furie R, Aroca G, Alvarez A, Fragoso-Loyo H, Zuta Santillan E, Rovin B, Brunetta P, Schindler T, Hassan I, Cascino M, Garg J, Malvar A. Two-Year Results from a Randomized, Controlled Study of Obinutuzumab for Proliferative Lupus Nephritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/two-year-results-from-a-randomized-controlled-study-of-obinutuzumab-for-proliferative-lupus-nephritis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/two-year-results-from-a-randomized-controlled-study-of-obinutuzumab-for-proliferative-lupus-nephritis/